Abstract
Background: Platelet factor 4/heparin (PF4/H) antibody detection is widely used to evaluate the risk of thrombosis in patients undergoing hemodialysis (HD). Most patients who are PF4/H-antibody-positive can survive thrombosis, but the reason has not been clarified. In addition, no valid preventive methods for thrombosis in patients undergoing HD have been confirmed. Methods: A single-center, semi-randomized controlled study was designed. In total, 157 patients fulfilled the inclusion criteria and participated. Patients were first divided according to PF4/H antibody detection and then subdivided randomly according to different anti-platelet agent descriptions. Results: (1) PF4/H antibody-positive patients suffered a significantly higher incidence of thrombosis than those who were antibody-negative; (2) PF4/H antibody-positive patients who survived a thrombosis manifested a significantly longer bleeding time and decreased maximum percentage of platelet aggregation inhibition; (3) aspirin and clopidogrel decreased the incidence of thrombosis in PF4/H antibody-positive patients by inhibiting platelet activation. Conclusion: The PF4/H antibody was effective for prediction of the risk of thrombosis, except in patients with dysfunctional platelets; aspirin manifested effects similar to clopidogrel in terms of prevention of thromboses in PF4/H antibody-positive patients, but costs much less and is therefore recommended.
Introduction
Thrombosis is common in patients undergoing hemodialysis (HD) and is viewed as a main complication that heavily influences patient prognosis. A recent clinical study showed that an arteriovenous fistula (AVF) embolism is the most frequent vascular complication in Chinese patients undergoing maintenance HD.Citation1 Schwab et al. reported that AVF embolism is a leading cause of AVF and transplant graft failure.Citation2 Myocardial infarction and cardiovascular diseases are considered the principal causes of high mortality in Chinese patients undergoing HD. Therefore, a risk evaluation for thrombosis and a prevention method are needed.
Heparin-induced thrombocytopenia (HIT) is a serious complication of heparin therapy, and is an immune-mediated syndrome caused by antibody against the platelet factor-4/heparin (PF4/H) complex that is characterized by thrombocytopenia and a high risk for thrombosis.Citation3,Citation4 Only a subset of PF4/H antibody-positive patients with classic HIT manifest thrombocytopenia, and only a portion of these develop thromboses.Citation5,Citation6 It has been reported that 28.5% of PF4/H antibody-positive patients undergoing HD suffered thromboses in a follow-up, whereas only 8.7% of those without the antibody suffered thromboses.Citation7 However, the association between the PF4/H antibody and thrombosis is equivocal. Another interesting phenomenon observed in clinical practice is that most patients undergoing HD with extremely high levels of the PF4/H antibody do not develop any thromboses, but the mechanism is unclear.
The most commonly used anti-platelet agents, aspirin and clopidogrel, prevent vascular embolisms in some patients. A recent multi-center, large-sample clinical trial indicated that treatment with aspirin plus dipyridamole has a significant preventative effect on AVF thrombosis in patients undergoing HD.Citation8 Furthermore, a randomized, double-blind placebo-control study found that clopidogrel reduces the frequency of early thrombosis in patients with a new AVF.Citation9 Anti-platelet therapy is valid for preventing myocardial and cerebral infraction in the general population, including patients undergoing HD; however, it is unclear whether the preventive effect occurs in the context of a high thrombosis risk.
This study was performed to address the following questions:
Is there an association between PF4/H antibody positivity and the incidence of thrombosis in patients undergoing HD?
Why do many patients undergoing HD who have extremely high PF4/H antibody titers not suffer from thrombotic events?
Can aspirin and clopidogrel successfully prevent thrombosis in patients undergoing HD and are there different thrombotic preventive effects between the two agents?
Methods
Study design and participants
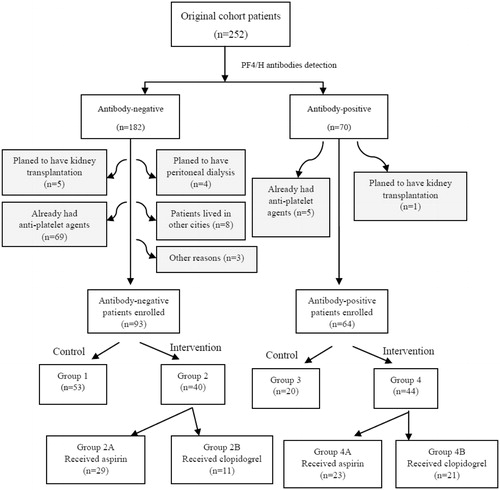
Our study was semi-randomized, controlled and interventional. In total, 252 patients undergoing maintenance HD in our HD Centre of the First Affiliated Hospital of Beidaihe Sanatorium of the Beijing Military Region were recruited. Before beginning the study, all patients were confirmed to be PF4/H antibody-positive with an enzyme-linked immunosorbent assay (ELISA) kit (GTI PF4 Enhanced, GTI Diagnostics, Waukesha, WI). Inclusion criteria were (1) undergoing HD for at least 3 months, (2) plan on receiving HD at the centre for ≥2 years, (3) no plan for a transplantation or other substitute therapy unless necessary, (4) no thrombocytopenia for at least 5 years until exposed to anticoagulants, (5) did not receive any anti-platelet agents before enrolment in our study and (6) provided written informed consent. Totally 95 patients were excluded from our study: 74 patients already had anti-platelet agents before the starting point; 6 patients receiving HD therapy are preparing for kidney transplantation in the near future (1 antibody-positive and 5 antibody-negative cases); 4 antibody-negative patients with certain residual urine volume merely received temporary HD and then underwent peritoneal dialysis instead; 8 antibody-negative patients from another cities were not able to undergo dialysis therapy in our centre for a long time; 3 antibody-negative cases were lost for another reasons. Finally, 157 patients (62.3%) fulfilled all inclusion criteria and participated. First, all enrolled patients were divided into anti-positive and anti-negative groups. Then all anti-positive patients were subdivided into three groups by randomly selecting ID numbers of patients using the statistic software CHISS (Chinese High Intellectualized Statistical Software, ver. 6.0, developed by Statistic Department of General Hospital of PLA, Beijing, China). All anti-negative patients were divided into three groups by the same method. Six groups were described as follows: group 1, antibody-negative and refused anti-platelet therapy (n = 53); group 2A, antibody-negative and taking aspirin (n = 29); group 2B, antibody-negative and taking clopidogrel (n = 11); group 3, antibody-positive and refused anti-platelet therapy (n = 20); group 4A, antibody-positive and taking aspirin (n = 23); group 4B, antibody-positive and taking clopidogrel (n = 21) (). HD characteristics and laboratory examinations were gathered at the beginning of the study, and blood samples were collected. Patients had their blood checked monthly from December 2009. Platelet counts and D-dimer levels were recorded from December 2009 to May 2011. Bleeding time (BT), maximum percentage of platelet aggregation inhibition (IPA-Max%) and PF4 were measured at the start, and PF4 also at the end, of the study. All associated information was collected, and a database was established using Microsoft Excel 2003 for Windows.
Dialysis and heparinization schedule
The main intradialytic blood flow (QB) was 290 ± 20 mL/min, the dialysis flow (QD) was 500 mL/min and the ultrafiltration rate was maintained near 0 during the checking times. One hundred and sixty-one out of 252 patients initially recruited had an AVF as the vascular access and 113 patients with an AVF finally completed the follow-up. The other 44 individuals used semi-permanent dialysis catheter as the vascular access. All patients underwent bicarbonate HD with a polysulfone low-flux filter (Fresenius HPS, Fresenius SE & Co. KGaA, Bad Homburg, Germany, hollow fiber dialyzer, 1.8 m2 surface area, Kuf 10 mL/h/mmHg). The anticoagulation schedules were described as follows: (a) UFH 1500 IU on the starting dialysis and 1500 ± 500 IU in continuous intradialytic infusion; (b) LMWH 50–75 IU/kg on the starting dialysis.
Blood sample detection and BTmeasurement
Blood samples from the 157 patients were collected in December 2009, before HD.
PF4/H antibody levels were measured in all samples by ELISA. The titer was considered positive when the optical density (OD) value was >0.4 absorbance units, indeterminate when the OD was 0.3–0.4 and negative when the OD was <0.3. Indeterminate PF4/H ELISA samples were considered negative in the statistical analysis. The PF4/H ELISA detected the IgA/IgM and IgG PF4/H antibodies. A portion of the blood sample was drawn into CTAD tubes (Vacutainer CTAD; Becton Dickinson, Plymouth, UK), cooled on ice and centrifuged for 20 min at 3500 rpm. PF4 titers were determined using commercially available sandwich ELISA kits (Asserachrom PF4; Diagnostica Stago, Asnieres, France).
Venous blood was drawn and mixed with sodium citrate. Platelet-rich plasma (PRP) was obtained by centrifugation at 2500 rpm for 10 min at 20 °C, and platelet-poor plasma (PPP) was obtained by centrifugation at 8500 rpm for 15 min at 20 °C. PRP was adjusted with autologous PPP (PLT, 280–300 × 109/L). Whether erythrocytes or leukocytes were observed in the PRP by light microscopy, a second centrifugation was conducted to minimize cell numbers (8500 rpm for 5 min). Light transmission aggregometry (LTA) of the PRP was performed in a double-channel Lumi-Aggregometer (Chrono-log Corp., Havertown, PA). We set the light transmission level to 15% for PRP and 85% for PPP. Aggregating agent (adenosine diphosphate, 4 μM) was added to the PRP in the aggregometer at 37 °C with persistent stirring at 1000 rpm. The percentage of inhibited platelet aggregation was expressed as the maximum percent change in light transmission from baseline by PPP – 100%.
To measure BT, a disposable Surgicutt device for adults (ITC, Thoratec Co., Edison, NJ) was applied to make a standard incision (4-mm long and 1.5-mm deep) on the volar surface of the left forearm, perpendicular to the antecubital crease, while maintaining a pressure of 45 mmHg on a sphygmomanometer cuff. The time until complete cessation of bleeding was determined to be the BT.
To determine platelet count, ∼2 mL of venous blood was collected from the antecubital vein into ethylenediaminetetraacetic acid-coated tubes and analyzed after 2 h using a Sysmex XE-2100D Automated Hematology Analyser (Sysmex America, Inc., Mundelein, IL). Platelet counts of all patients were measured once per month. STAGO-Liatest D-dimer kits were used to quantitate plasma D-dimer titers by the immune-turbidimetric method using a STAGO analyzer. Patients who participated in the study had their D-dimer titers measured monthly.
Clinical outcome definition
The endpoint of the present study was all-cause thrombotic events. The incidence of thrombosis within the 18-month follow-up period was determined by reviewing all medical charts, including myocardial infarction, cerebral infraction, AVF embolism and semi-permanent dialysis catheter embolism. Diagnosis of a thrombotic case required clinical symptoms, imaging evidence (computed tomography, magnetic resonance imaging or Doppler flow imaging) and operative findings, if necessary.
Statistical analyses
Dichotomous variables are expressed as ratios, and continuous variables as means (standard deviations) or medians (quartiles). Dichotomous variables were compared with the χCitation2 test, and continuous variables were compared by the t test, Mann–Whitney U-test or Wilcoxon’s rank-sum test, as appropriate. Proportions were compared using Pearson’s χCitation2 test or Fisher’s exact probability test. Thrombotic event-free survival curves were created by the Kaplan–Meier method, and the log-rank test was applied to test the statistical significance of thrombosis among groups based on the survival curves. A Cox proportional hazard model was applied to analyze the effects of the PF4/H antibody, anti-platelet agent use and other potential risk factors on the time to thrombosis. Data were analyzed using the SPSS software ver. 16.0 (SPSS, Inc., Chicago, IL). A p value <0.05 was considered significant.
Results
Seventy of the 252 (27.8%) patients were PF4/H antibody-positive. Finally, 157 patients (64 antibody positive vs. 93 antibody negative cases) participated in the study. shows a comparison of the clinical characteristics of antibody-positive and -negative patients. General characteristics (including age, gender, weight, systolic blood pressure and diastolic blood pressure), HD parameters (including Kt/v, PCR and heparin prescription), laboratory examinations (including Cr, ALB, Ca, P, PTH, PLT and Hb), pathogens of renal failure (including chronic glomerulonephritis, diabetes nephropathy, hypertension, polycystic kidney and others) and past thrombotic events (including myocardial or cerebral infraction) were not significantly different between PF4/H antibody-positive and -negative patients. Duration and total HD hours per week in the PF4/H antibody-positive patients was significantly greater than those in the PF4/H antibody-negative patients. Time-averaged concentration urea and baseline D-dimer titers were both significantly different between the PF4/H antibody-positive and negative groups.
Table 1. Characteristics of platelet factor 4/heparin (PF4/H) antibody-positive and negative patients.
In total, 37 patients suffered a thrombosis within the 18-month follow-up period. shows a comparison of the clinical and dialytic characteristics of patients with and without thrombosis during follow-up period. Age, gender, duration of HD, time of HD per week, anticoagulant choice and some dialysis parameters (including Kt/v, TAC urea and PCR) are all similar between two groups (p all >0.05). However, the incidence of thrombosis in the PF4/H antibody-positive patients was significantly higher than that in PF4/H antibody-negative patients (34.4% vs. 16.1%, p = 0.007). All causes of thrombotic events in PF4/H antibody-positive and -negative patients are manifested in .
Table 2. Characteristics of patients with and without thrombotic events in follow-up.
Table 3. Comparison of the incidence of thrombosis between PF4/H antibody-positive and antibody-negative patients (using Fisher exact probability test in 2 × 2 tables).
The cumulative incidence of thrombocytopenia increased significantly in group 3 compared with that in the other five groups (p = 0.002), but no significant difference was observed among these groups (). The platelet counts at the 18-month follow-up in group 2B were the highest, and they were the lowest in group 3 among all six groups. Mean and maximum D-dimer titers were significantly higher in groups 3 and 4A than those in the other four groups, and they were the highest in group 3. Minimal differences in D-dimer titers were observed among groups 1, 2A, 2B and 4B (p-values were all above 0.05 for mean and maximum by Kruskal–Wallis test). PF4 titers measured in PF4/H antibody-positive patients were significantly higher than those in PF4/H antibody-negative patients (48.18 ± 11.55 vs. 28.94 ± 7.36 IU/mL, p < 0.001). Group 3 showed the highest PF4 titers among the six groups (p < 0.001) ().
Figure 2. The cumulative frequency of thrombocytopenia was significantly higher in group 3 than in the other five groups. The cumulative frequency of thrombocytopenia in the other five groups was approximated using the Gehan–Breslow–Wilcoxon test.
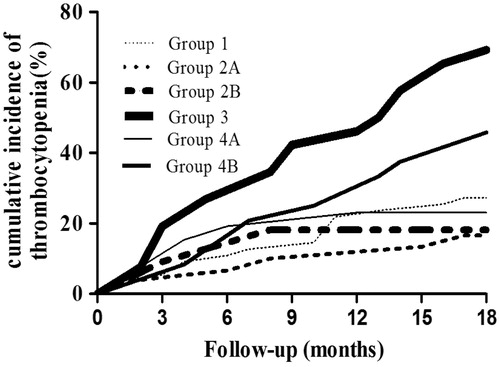
Table 4. Comparison of platelet countsa, D-dimer titersa and platelet factor 4 (PF4)b at the follow-up.
Patients in groups 4A and 4B underwent significantly fewer thrombotic incidents than those in group 3, but no significant difference was observed between groups 4A and 4B. No significant differences were found among groups 1, 2A or 2B (). We introduced PF/H antibody positivity, aspirin and clopidogrel use, gender, age, dialysis hours per week, duration of dialysis, anticoagulants (UFH/LMWH) and past thrombotic events as variables in the Cox proportional hazards regression model. Except for PF4/H antibody positivity and anti-platelet agents use, the other factors were not significantly associated with thrombosis (). Aspirin and clopidogrel both apparently played an important role in the context of high PF4/H antibody levels, and the preventive effects of clopidogrel were more evident than those of aspirin (hazard ratios for thrombosis were 0.259 and 0.406, respectively).
Figure 3. (A) Of the antibody-positive groups, thrombosis-free survival rates 4A and 4B are significantly higher than 3. (B) Of the antibody-negative groups, patients in groups 1, 2A and 2B manifested a similar incidence of thrombosis. Analyzed by log-rank survival test.
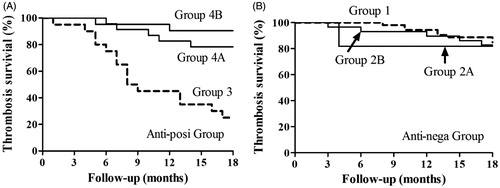
Table 5. Cox proportional hazards regression model for thrombotic events.
Groups 1 and 3 patients both refused anti-platelet agents at the beginning of the study. These two groups were subdivided into four subgroups based on whether they suffered thrombotic events during follow-up. We compared the four subgroups in terms of BT, IPA-Max% and beginning PF4 titers. Patients in group 3, who survived a thrombosis, manifest significantly longer BT and lower IPA-Max% compared with patients who had suffered a thrombosis (p = 0.001). PF4 titers are approximate between two subgroups. However, minimal differences were observed in group 1 patients ().
Table 6. Comparison of parametersa between PF4/H antibody positive and negative groups surviving from and suffering thrombotic events.
Discussion
Thrombotic events are the most severe complication and a main cause of the high mortality rate in patients undergoing HD.Citation10,Citation11 Thus, it is necessary to establish a reliable and accurate method to evaluate the possibility of thrombosis in high-risk patients. Detecting the PF4/H antibody has been widely used to estimate the prognosis of patients with HIT.Citation7,Citation12 However, some studies have not supported an association between the PF4/H antibody and thrombosis.Citation13,Citation14 In our study, PF4/H antibody detection was reliable for prediction of future thrombotic events, which was consistent with previous surgical studies.Citation15,Citation16 There is a question as to why many PF4/H antibody-positive patients undergoing HD survive thrombotic events. We speculate that activation of platelets plays a key role in thrombosis; thus, we evaluated platelet status. BT is an invasive technique with a low sensitivity for mild or moderate abnormalities of inherited platelet defects, but this situation may be completely different when different agents are prescribed.Citation17,Citation18 However, BT was carried out by experienced personnel in our study and was a useful technique to evaluate the in vivo effects of the anti-platelet agents. LTA is also useful for evaluation of platelet status, although it was reported to be poorly reproducible in some studies; however, it remains the gold standard test of platelet function.Citation19,Citation20 PF4 particles are stored in platelets and released upon their activation. The level of PF4 particles parallels the degree of platelet activation to a certain extent. We found that some PF4/H antibody-positive patients who survived a thrombosis had a significantly longer BT and decreased IPA-Max, suggesting that a platelet dysfunction had occurred. When platelets are widely activated by the PF4/H-antibody complex, platelet-derived microparticles with coagulant activity are released into the blood and thrombosis subsequently develops.Citation21 However, malfunctioning platelets cannot be activated effectively even when exposed to high PF4/H antibody titers. We provided clinical evidence for the above question. Anti-platelet agents could render platelets dysfunctional but could anti-platelet agents prevent thrombotic events by affecting platelet activation when exposed to high PF4/H antibody titers?
Clopidogrel and aspirin are the most popular anti-platelet agents in mainland China and selectively inhibit a single platelet activation pathway.Citation22–24 However, the effect of these two agents is not uniform; indeed, some patients do not benefit. Clopidogrel is a potent and specific inhibitor of the platelet P2Y12 receptor, whereas aspirin inhibits thromboxane biosynthesis by inactivating platelet cyclooxygenase-1.Citation25–27 Both aspirin and clopidogrel prevent AVT thrombosis in patients undergoing HD,Citation28 but a combination of both is associated with a significantly increased risk of bleeding that does not result in a lower frequency of graft thrombosis.Citation29 Patients in our study did not receive combined anti-platelet therapy (aspirin plus clopidogrel) for serious bleeding events. We found that patients with low PF4/H antibody titers did not benefit from either aspirin or clopidogrel to prevent thrombosis. However, patients with a high PF4/H antibody titer who refused aspirin or clopidogrel suffered significantly more thrombotic events compared with those receiving aspirin or clopidogrel. Patients who were PF4/H antibody positive and who received anti-platelet therapy showed significantly decreased levels of PF4, which suggested that platelets in these patients were incompletely activated. Platelet counts were used to evaluate platelet status and, indirectly, the decreased incidence of thrombocytopenia suggested that aspirin and clopidogrel prevented PF4/H antibody-induced thrombosis by inhibiting platelet activation. Based on our results, patients with a high PF4/H antibody titer should take anti-platelet agents as early as possible to prevent thrombosis. Notably, the cost of clopidogrel is higher than that of aspirin (Renminbi (Chinese currency, RMB) 14.0 vs. 0.35 yuan per tablet, respectively); however, clopidogrel showed no additional advantage. Therefore, aspirin is recommended.
We found that the PF4/H antibody was a strong risk factor for thrombosis, but its effects were much weaker in patients with a platelet malfunction; aspirin was cheap but provided equipotent prevention as clopidogrel when patients developed high PF4/H antibody titers. Of course, our study had some limitations. First, our results were based only on a clinical study, and the conclusions need be confirmed in vitro. Furthermore, relatively higher positivity of PF4/H antibodies (28%) was detected in our patients compared with some published papers and we did not much confirmed that its effect on the results. Third, we had lost many patients at the first beginning and most of them were PF4/H antibody negative patients, which might lead to imbalance of cohort structure. Besides, it was semi-randomized study. Thus, a larger-scale study with longer term observations will be necessary.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
The authors thank Deyang Kong, PhD and Lirong Hao, PhD of the First Affiliated Hospital of Harbin Medical University for help with the study design and data analysis. Some important analytical equipment used in this study was graciously provided by the First Affiliated Hospital of Harbin Medical University.
References
- Yu Q, Yu H, Chen S, Wang L, Yuan W. Distribution and complications of native arteriovenous fistulas in maintenance hemodialysis patients: a single-center study. J Nephrol. 2011;24:597–603
- Schwab SJ, Harrington JT, Singh A, et al. Vascular access for hemodialysis. Kidney Int. 1999;55:2078–2090
- Chang JJ, Parikh CR. When heparin causes thrombosis: significance, recognition, and management of heparin-induced thrombocytopenia in dialysis patients. Semin Dial. 2006;19:297–304
- Greinacher A, Althaus K, Krauel K, Selleng S. Heparin-induced thrombocytopenia. Hamostaseologie. 2010;30:17–18, 20–28
- Kelton JG. Heparin-induced thrombocytopenia: an overview. Blood Rev. 2002;16:77–80
- Warkentin TE. Heparin-induced thrombocytopenia: a clinicopathologic syndrome. Thromb Hemost. 1999;82:439–447
- Mureebe L, Coats RD, Silliman WR, Shuster TA, Nichols WK, Silver D. Heparin-associated antiplatelet antibodies increase morbidity and mortality in hemodialysis patients. Surgery. 2004;136:848–853
- Dixon BS, Beck GJ, Vazquez MA, et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191–2201
- Dember LM, Beck GJ, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299:2164–2171
- Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med. 2002;137:563–570
- Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562
- Pena de la Vega L, Miller RS, Benda MM, et al. Association of heparin-dependent antibodies and adverse outcomes in hemodialysis patients: a population-based study. Mayo Clin Proc. 2005;80:995–1000
- lackCarrier M, Knoll GA, Kovacs MJ, Moore JC, Fergusson D, Rodger MA. The prevalence of antibodies to the platelet factor 4-heparin complex and association with access thrombosis in patients on chronic hemodialysis. Thromb Res. 2007;120:215–220
- Alexy T, Tucker S, Boyle S, Rowe VL, Weaver FA, Liebman HA. Heparin-platelet factor 4 antibodies are frequent after vascular surgery but are not a frequent cause of graft thrombosis or thrombocytopenia. J Vasc Surg. 2008;48:377–381
- Mattioli AV, Bonetti L, Carletti U, Ambrosio G, Mattioli G. Thrombotic events in patients with antiplatelet factor 4/heparin antibodies. Heart. 2009;95:1350–1354
- Mattioli AV, Bonetti L, Zennaro M, Ambrosio G, Mattioli G. Heparin/PF4 antibodies formation after heparin treatment: temporal aspects and long-term follow-up. Am Heart J. 2009;157:589–595
- Lordkipanidze M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J. 2007;28:1702–1708
- Harrison P, Frelinger AL 3rd, Furman MI, Michelson AD. Measuring antiplatelet drug effects in the laboratory. Thromb Res. 2007;120:323–336
- Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303:754–762
- Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol. 2004;24:1980–1987
- Bevers EM, Comfurius P, Zwaal RF. Platelet procoagulant activity: physiological significance and mechanisms of exposure. Blood Rev. 1991;5:146–154
- Gurbel PA, Tantry US, Shuldiner AR, Kereiakes DJ. Genotyping: one piece of the puzzle to personalize antiplatelet therapy. J Am Coll Cardiol. 2010;56:112–116
- Kozinski M, Bielis L, Wisniewska-Szmyt J, et al. Diurnal variation in platelet inhibition by clopidogrel. Platelets. 2011;22:579–587
- Kozinski M, Bielis L, Wisniewska-Szmyt J, et al. Increased morning ADP-dependent platelet aggregation persists despite dual antiplatelet therapy in patients with first ST-segment elevation myocardial infarction: preliminary report. Cardiol J. 2008;15:530–536
- Michelson AD, Cattaneo M, Eikelboom JW, et al. Aspirin resistance: position paper of the Working Group on Aspirin Resistance. J Thromb Hemost. 2005;3:1309–1311
- Kasprzak M, Kozinski M, Bielis L, et al. Pantoprazole may enhance antiplatelet effect of enteric-coated aspirin in patients with acute coronary syndrome. Cardiol J. 2009;16:535–544
- Barton JF, Hardy AR, Poole AW, Mundell SJ. Reciprocal regulation of platelet responses to P2Y and thromboxane receptor activation. J Thromb Hemost. 2008;6:534–543
- Cannon CP, Rhee KE, Califf RM, et al. Current use of aspirin and antithrombotic agents in the United States among outpatients with atherothrombotic disease (from the REduction of Atherothrombosis for Continued Health [REACH] Registry). Am J Cardiol. 2010;105:445–452
- Kaufman JS, O'Connor TZ, Zhang JH, et al. Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol. 2003;14:2313–2321