Abstract
Renal stone disease and gallstone disease are widely prevalent and costly disease across the globe. Both renal stone disease and gallstone disease are associated with a variety of diseases including obesity, metabolic syndrome, dyslipidemia, hypertension, insulin resistance diabetes and gout. Importantly, the presence of either renal stone disease or gallstone disease is associated with an increased risk of cardiovascular disease. In a recent study of the Atherosclerosis Risk in Communities (ARIC), individuals with a history of gallstones were 54% more likely to report a history of nephrolithiasis after adjusting for age, gender, body size and other factors. Furthermore, in three large cohorts including over 240,000 subjects: the Nurses’ Health Studies (NHS) I and II and the Health Professionals Follow-up Study (HPFS), showed that gallstone disease is independently associated with nephrolithiasis. The mechanisms linking gallstone disease and renal stone disease are complex and not yet established. Insulin resistance, lithogenic diets, alterations of transporters in gallbladder and urinary system, and pH are possible potential mechanisms for future exploration. How the liver communicates with kidney in individuals with renal stone disease and gallstone disease is not well known and whether this communication is similar as in hepto-renal syndrome is subject for future research. Further research is needed to determine: (i) the underlying mechanisms of renal stone disease and gallstone disease; (ii) the potential treatment of renal stone disease and gallstone disease.
Introduction
Gallstone disease and renal stone disease are common, well-recognized and widely prevalent conditions across the globe. The prevalence of gallstones are estimated to be 10–15% of adults,Citation1 while the prevalence of kidney stone disease worldwide is estimated to be around 2–20%, and common stone is calcium oxalate.Citation2–4 Gallstones are classified as either cholesterol (80–90%) or pigment stones (10–20%). Cholesterol stones are formed as long-term cholesterol supersaturated bile, whereas pigment stones are mainly due to polymerized calcium bilirubinate. Most asymptomatic gallstone carriers require no therapy.Citation5 Gallstone disease and renal stone disease are associated with obesity, type 2 diabetes mellitus, dyslipidemia and hyperinsulinemia.Citation6,Citation7 These metabolic conditions lead to renal stone formation due to an increased formation of uric acid. The formation of cholesterol gallstones is thought to be associated with an increased hepatic secretion of cholesterol into bile due to the presence of these metabolic disorders.Citation8 The only established dietary risk for cholesterol gallstone is a high caloric intake and the distribution of gallstones in different populations appear to be related to high dietary intake of cholesterol and fats (Western diets). Other modifiable risk factors for gallstone and renal stone disease are obesity, metabolic syndrome and rapid weight loss. Importantly the incidence of both gallstones and renal stone disease increases with age.Citation8 The development of diabetes, hypertension and gout are associated with a marked risk of developing chronic kidney disease (CKD) in individuals with chronic renal disease.Citation9 Due to epidemic of diabetes and obesity, it is expected that the prevalence of cholesterol gallstone and renal stone disease will increase and this will add more to burden of health care authorities. Therefore, life style management and weight loss are strongly recommended to combat the epidemic of obesity and metabolic syndrome.
Renal stones can be due to different causes and majority could be due to idiopathic causes (genetic-idiopathic hypercalcemia). Other causes include metabolic and inherited disorders (cystine stones) and anatomical disorders with or without chronic urinary tract infection (urinary tract infection likely struvite stone-calcium, magnesium, ammonium phosphate).
The association between cholesterol gallstone and renal stone disease is of considerable importance as each condition is associated with high risk of cardiovascular disease (CVD) and mortality. Ruhl and EverhartCitation10 showed an increase in association between cholesterol gallstone and CVD and increase in risk of mortality. Furthermore, in subjects with ultrasound evidence of gallstones there was an increase in the proportion of subjects with coronary heart disease, increased carotid artery intima-media thickness (CIMT).Citation11 The cardiovascular effect of gallstones appeared to be independent of the features of metabolic syndrome.Citation12 Importantly, the presence of renal stones disease is associated with an increased risk for MI, and this risk is independent of CKD, hypertension, diabetes, obesity, dyslipidemia, gout, alcohol dependence and tobacco use.Citation13 Therefore, subjects with gallstones and renal stone disease are most likely at an increased risk of overall mortality and CVD (). The focus of this review is about the association between renal stone disease and cholesterol gallstone. Both renal stone disease and cholesterol gallstone are associated with insulin resistance and metabolic syndrome, diabetes and dyslipidemia; therefore, the subsequent sections of this review will discuss the association between renal stone disease and cholesterol gallstone and insulin resistance, metabolic syndrome, diabetes and dyslipidemia.
Figure 1. The complex relationship between metabolic syndrome, renal stone disease and gallstones diseases. How the liver communicates with kidney in individuals with renal stone disease and gallstone disease is not well known. The presence of renal stone disease or gallstone per se should lead to an additional evaluation of modifiable metabolic risk factor that leads to an increase in the risk of CVD GD.
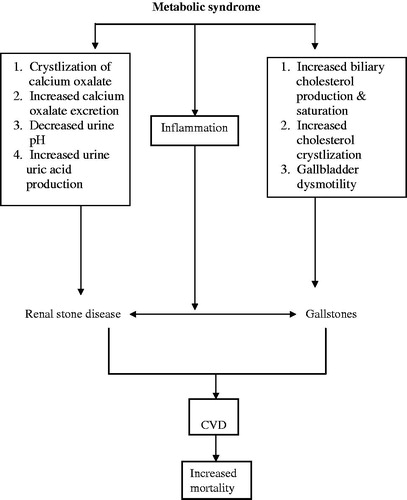
The association between cholesterol gallstone and renal stone disease
The association between the presence of renal stone disease and cholesterol gallstones have been reported in different studies. For instance, Taylor et al. showed that in more than 240,000 participants followed for 14 to 24 years in USA, the multivariate odds ratio of kidney stone history in individuals with gallstone history compared to those without was 1.65 (95% CI 1.46–1.86) in older women, 1.85 (95% CI 1.65–2.07) in younger women and 1.61 (95% CI 1.41–1.85) in men. Prospectively, the multivariate relative risk of incident kidney stones in participants with gallstone history compared to those without was 1.26 (95% CI 1.09–1.44) in older women, 1.32 (95% CI 1.14–1.52) in younger women and 1.28 (95% CI 1.03–1.57) in men. The multivariate relative risk of incident gallstones in participants with kidney stone history compared to those without was 1.17 (95% CI 1.06–1.29) in older women, 1.31 (95% CI 1.19–1.45) in younger women and 1.51 (95% CI 1.35–1.68) in men. Prospective lag analyses instituting a delay of 4 years between the diagnoses of gallstones and kidney stones yielded similar results. The authors concluded that gallstones and kidney stones are independently associated.Citation14 Gallstones are regarded as risk factor for renal stone disease. Akoudad et al.Citation15 [study of the Atherosclerosis Risk in Communities (ARIC) cohort-USA] showed that in multivariable adjustment, prevalent kidney stone disease was significantly (p < 0.05) associated with male gender (PR = 2.50), increased serum triglycerides (PR = 1.07 per SD increase), diabetes (PR = 1.27) and gallstone disease (PR = 1.54). This study also showed that significant interactions with race, older age, higher triglycerides and gallbladder disease showing stronger associations with kidney stones in African–Americans compared to whites, and male gender showing a stronger association with kidney stone disease in whites. Male gender, hypertension and diabetes were identified as correlates of incident kidney stone disease-related hospitalizations. Li et al.Citation16 used registry of the Taiwan National Health Insurance Research Database (NHIRD) of the 23.74 million people in the database, and followed 25,258 gallstone patients (54.5% female) and 101,029 control patients. They found that the risk of developing renal stones was 1.68-fold greater in gallstone patients, compared with patients without gallstones after adjusting for age, sex and comorbidities. The substantially increased risk of renal stones was also significant in gallstone patients regardless of comorbidities. In the follow-up period <1 year, the adjusted hazard ratios was 2.51 (95% CI = 2.25–2.80) compared to the non-gallstone group. The cumulative incidence of renal stone in the gallstone group was higher than the non-gallstone group (p < 0.0001, in the log-rank test). This large cohort study demonstrated that the risk of renal stones is significantly higher in gallstone patients, compared with the general population.
Lai et al.Citation17 showed that the prevalence of gallbladder stones in Taiwanese patients with chronic kidney disease is significantly higher than in those without chronic kidney disease. Their findings suggest that increasing age, chronic kidney disease, body mass index ≥27 kg/m2, metabolic syndrome, and cirrhosis are the related factors for gallbladder stone formation. Furthermore, the risk of gallstone formation is higher in patients before dialysis.Citation18 Interestingly, Park et al.Citation19 showed that the risk of gallstone formation is higher in patients with renal transplant than those without transplant. Although further population studies are needed, it is possible to suggest that the presence of gallstone may suggest the need to check for renal stone disease and chronic kidney disease.
Obesity and renal stone disease and cholesterol gallstones
Obesity (BMI > 30 kg/m2) is an important risk factor in the pathogenesis of both renal stone disease and cholesterol gallstones. The current obesity epidemic across the globe is associated with a significant increase in morbidity and mortality. Importantly, there is an association between obesity and significant increase in renal diseases and cholesterol gallstones. In Korean post-menopausal women, there is a significant association between the occurrence of gallbladder stones and age, obesity, abdominal obesity and insulin resistance.Citation20 Insulin resistance and low high-density lipoprotein-cholesterol were found to be an independent predictor of gallbladder stone formation in women.Citation21 Furthermore, successful treatment of obesity leads to a significant decrease in the progression for renal diseases.Citation22 The features of the metabolic syndrome (insulin resistance, dysglycemia, dyslipidemia, hypertension and central obesity) are not only risk factors for cardiovascular disease but also for renal disease and cholesterol gallstone.Citation23,Citation24 Obesity may be associated with an end-stage renal disease (ESRD), diabetes, hypertension and nephrotic syndrome.Citation25 Epidemiologic studies have shown that obesity is also associated with an increased risk of renal stone formation.Citation26–28 Abu Ghazaleh and BudairCitation29 showed that among 8346 patients with renal stones disease, 68% were obese and overweight. Interestingly, further increase in body mass index more than 30 kg/m2 was found not to be associated with further increases in the risk of renal stone disease.Citation30
Cholesterol gallstones and renal stone disease and metabolic syndrome
Insulin resistance and metabolic syndrome are important features of pathogenesis of gallstone and renal stone disease. Chen et al.Citation31 showed that metabolic syndrome is associated with cholesterol gallstone and the more the increase in components of metabolic syndrome the more the risk of higher prevalence cholesterol gallstones. Tsai et al.Citation32 showed that the presence of a significant association between abdominal adiposity and the incidence of symptomatic gallstone disease is independent of body mass index. Interestingly, West et al.Citation33 showed with an increase in the components of the metabolic syndrome there is an increase in the incidence of renal stone diseases. Jeong et al.Citation34 showed that metabolic syndrome is associated with a significantly increased risk of kidney stone development. The association between insulin resistance/metabolic syndrome and gallstone are also confirmed in other studies.Citation35–37 Nervi et al.Citation38 confirmed the association between insulin resistance, metabolic syndrome, fatty liver and risk of gallstones. Even in lean non-obese individual insulin resistance is associated with an increase in gallstone formation. Chang et al.Citation39 showed that the prevalence of obesity, abdominal obesity and metabolic syndrome in the subjects with gallstones were higher than in those without. Insulin resistance proved to be independent predictors of gallstones. Importantly, Nakeeb et al.Citation40 concluded that insulin resistance alone may be responsible for gallbladder dysmotility that may lead to gallstone formation.
Metabolic syndrome is associated with excess nutritional intake of lithogenic substances such as calcium, oxalate and uric acid. Metabolic syndrome, commonly associated with obesity, alters renal acid–base metabolism, resulting in a lower urine pH and an increased risk of uric acid stone disease.Citation41,Citation42 The low urine pH is caused by deficient ammonia production, which appears to be related to insulin resistance. Bariatric surgery and antiobesity medication (orlistat) are associated with a risk of hyperoxaluria and a potential risk of associated stone formation and oxalate nephropathy. Furthermore, low-carbohydrate diets increase the risk of both calcium and uric acid stones.Citation43,Citation44 Therefore, it is possible to conclude that metabolic syndrome and treatment of metabolic syndrome are associated with an increase in risk of renal stone disease.
Cholesterol gallstones and renal stone disease and diabetes
Large number of studies showed a link between renal stone disease and type 2 diabetes mellitus as well the risk of diabetes is increased in those individual with renal stone disease. In a cross-sectional study of three large cohorts including over 200,000 participants, it was concluded that diabetes is a risk factor for the development of kidney stones.Citation45 This may be in part explained by the fact that metabolic syndrome is associated with a calcium oxalate crystal deposit.Citation46 In addition, metabolic syndrome is also associated with a decrease in urinary pH and degree of insulin resistance, which creates favorable condition for stone formation.Citation41,Citation47 Furthermore, one renal manifestation of insulin resistance is low urinary ammonium and pH and this leads to an increased risk of uric acid precipitation despite normouricosuria.Citation48,Citation49
Interestingly, stone formers in patients with type II diabetes mellitus excrete significantly greater urinary oxalate and significantly lower urine pH than those without diabetes mellitus.Citation50 Diabetes is also linked with a risk for uric acid stone.Citation51 Daudon et al.Citation52 showed that the proportion of patients with type 2 diabetes was significantly higher among uric stone than among calcium stone formers (27.8 vs. 6.9%; p < 0.0001). Stepwise regression analysis identified type 2 diabetes as the strongest factor that was independently associated with the risk for uric acid stones (odds ratio 6.9; 95% confidence interval 5.5 to 8.8). Therefore, patients with uric acid stones and overweight should be screened for diabetes and components of the metabolic syndrome. Another rare complication associated with diabetes and renal stone disease is emphysematous pyelonephritis (EPN), which is a severe acute necrotizing infection of the renal parenchyma and perirenal tissue, characterized by gas formation and can be fatal if not early diagnosed. 90% of cases are seen in association with diabetes mellitus and may be due to renal stone disease. Several case reports showed the link between EPN and diabetes and renal stone disease.Citation53–55
Diabetes mellitus and gall bladder stones are closely linked costly diseases. Several studies from around the world reported an increased prevalence of gallbladder stones in patients with diabetes mellitus. A study from New Zealand reported a gallbladder stones prevalence of 32.7% among diabetic patients as compared to 20.8% in controls.Citation56 Another study from Italy showed that the prevalence of gallstone disease is significantly higher in diabetic patients than in the general population (24.8 vs. 13.8%).Citation57 The prevalence of gallstone was 40% among Libyan diabetic individuals and correlate positively with age and obesity.Citation58 In Taiwan, the prevalence of gallstone was 14.4% and the risk increased with age and BMI.Citation59 Furthermore, in Iraq the prevalence was 33% of gallstones in type 2 DM increases and correlates positively with obesity in female with increased parity and long and uncontrolled diabetes.Citation60
Inflammation and renal stone disease and gallstone disease
Obesity was shown to be an independent risk factor for infection.Citation61,Citation62 Inflammation is associated with risk of gallstones. Inflammation is associated with high risk of cholesterol gallstones. Interestingly, both obesity and dietary carbohydrates increase gallbladder total fat, triglycerides and inflammatory mediators.Citation63 Inflammation is an important part of the metabolic syndrome, obesity, NAFLD and insulin resistance.Citation23,Citation64,Citation65 Obesity is also associated with a risk of urinary tract infection (UTI). The increase in body mass index (BMI) appears to be associated with an increased risk for UTI and pyelonephritis.Citation66 In a study by the Prostate Study Group of Australian Society for Urology, obesity was identified as risk factor for UTI.Citation67 In addition to the above factors, UTI may be another factor that leads to obesity-induced renal stone disease. Therefore, it is possible to suggest that the increased risk of infection with obesity may increase the risk not only of renal stone disease but also of gallstone disease.
Potential possible mechanism for the association between renal stone disease and gallstones disease
The mechanisms that explain the association between renal stone disease and gallstone are complex and not yet fully investigated. The following are attempts to speculate about potential mechanisms ().
High caloric intake and cholesterol and fats are risk factor for cholesterol gallstones and renal stone disease.Citation1–3
Insulin resistance which is a cornerstone in the pathogenesis of metabolic syndrome, may lead to excess biliary cholesterol production and saturation and gallbladder dysmotilityCitation1,Citation14,Citation24
Insulin resistance at the kidney level is associated with calcium oxalate crystal deposit. In addition, metabolic syndrome is also associated with a decrease in urinary pH and degree of insulin resistance, which creates favorable condition for stone formation. Furthermore, one renal manifestation of insulin resistance is low urinary ammonium and pH and this leads to an increased risk of uric acid precipitation despite normouricosuria.Citation41,Citation42
Obesity is regarded as an inflammatory condition. Inflammation may be the potential link between renal stone disease and gallstones. Inflammation is associated with insulin resistance.Citation63–65
It is not yet clear whether the gallstone disease is associated with channelopathy in renal tubule as well as in the biliary system. The liver and kidney communicate with each other and the best example is the hepato-renal syndrome.Citation68 Indeed, further research is needed to test such hypothesis.
Relevance of renal and gallstones in renal failure
Renal stone disease can be associated with different complications during renal failure (infection, progression of loss of renal function). Renal stone disease was also reported in renal transplant, in particular, during treatment with Cinacalcet.Citation69 Interestingly, the risk of gallstone formation is higher in patients before dialysis but not those undergoing chronic hemodialysis therapy, and in patients with renal transplant than those without transplant.Citation18,Citation19 However, Badalamenti et al.Citation70 reported the presence of gallstone in 28% of 119 patients on regular dialysis treatment and no relationships were found between gallstones and age or modes of dialysis. Therefore, screening for renal stone and gallstone disease during renal disease may allow early diagnosis and management.
Conclusion
Renal stone disease and cholesterol gallstones are common diseases across the globe. The risk of formation of cholesterol gallstones and renal stone disease markedly increased in individuals with obesity, metabolic syndrome, NAFLD and insulin resistance. The evidence started to emerge that gallstone disease is independently associated with nephrolithiasis. Further research is needed to confirm these observations. The underlying pathophysiology of the association of renal stone disease with gallstone disease and therapeutic interventions will be a subject for future researchers to explore. It is not yet clear whether treatment of the features of metabolic syndrome and insulin resistance will treat or decrease the incidence of renal stone disease and cholesterol gallstones. Different studies showed potential benefit of treatment of weight loss in treatment of renal stone diseases and this may well have potential benefit in treating cholesterol gallstones disease. Until specific treatment is established, the best practice will be to treat the features of the metabolic syndrome through weight loss and life style changes.
Declaration of interest
Authors declare no conflict of interest.
References
- Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511
- Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994;46:893–899
- Indridason OS, Birgisson S, Edvardsson VO, Sigvaldason H, Sigfusson N, Palsson R. Epidemiology of kidney stones in Iceland: a population-based study. Scand J Urol Nephrol. 2006;40:215–220
- Buchholz NP, Abbas F, Afzal M, Khan R, Rizvi I, Talati J. The prevalence of silent kidney stones – an ultrasonographic screening study. J Pak Med Assoc. 2003;53:24–25
- Marschall HU, Einarsson C. Gallstone disease. J Intern Med. 2007;261:529–542
- Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant. 2011;26:864–868
- Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–996
- Ahmed MH, Hamad MA, Routh C, Connolly V. Statins as potential treatment for cholesterol gallstones: an attempt to understand the underlying mechanism of actions. Expert Opin Pharmacother. 2011;12:2673–2681
- Saucier NA, Sinha MK, Liang KV, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis. 2010;55:61–68
- Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology. 2011;140:508–516
- Mendez-Sanchez N, Bahena-Aponte J, Chavez-Tapia NC, et al. Strong association between gallstones and cardiovascular disease. Am J Gastroenterol. 2005;100:827–830
- Mendez-Sanchez N, Zamora-Valdes D, Flores-Rangel JA, et al. Gallstones are associated with carotid atherosclerosis. Liver Int. 2008;28:402–406
- Rule AD, Roger VL, Melton III LJ, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641–1644
- Taylor EN, Chan AT, Giovannucci EL, Curhan GC. Cholelithiasis and the risk of nephrolithiasis. J Urol. 2011;186:1882–1887
- Akoudad S, Szklo M, McAdams MA, et al. Correlates of kidney stone disease differ by race in a multi-ethnic middle-aged population: the ARIC study. Prev Med. 2010;51:416–420
- Li CH, Sung FC, Wang YC, Lin D, Kao CH. Gallstones increase the risk of developing renal stones: a nationwide population-based retrospective cohort study. QJM. 2014 Jan 22. [Epub ahead of print]. doi: 10.1093/qjmed/hcu017
- Lai SW, Liao KF, Lai HC, Chou CY, Cheng KC, Lai YM. The prevalence of gallbladder stones is higher among patients with chronic kidney disease in Taiwan. Medicine (Baltimore). 2009;88:46–51
- Kazama JJ, Kazama S, Koda R, Yamamoto S, Narita I, Gejyo F. The risk of gallbladder stone formation is increased in patients with predialysis chronic kidney disease but not those undergoing chronic hemodialysis therapy. Nephron Clin Pract. 2009;111:c167–c172
- Park SH, Hahm JS, Seong SS, et al. Incidence of gallbladder stones in renal transplant recipients. Korean J Gastroenterol. 2004;44:42–46
- Kim SS, Lee JG, Kim DW, et al. Insulin resistance as a risk factor for gallbladder stone formation in Korean postmenopausal women. Korean J Intern Med. 2011;26:285–293
- Shebl FM, Andreotti G, Meyer TE, et al. Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer. 2011;105:1424–1429
- Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–1574
- Ahmed MH, Byrne CD. Metabolic syndrome, diabetes & CHD risk. In: Packard CJ, ed. The Year in Lipid Disorders. Oxford UK: Clinical Publishing; 2007:3–26
- Ahmed MH, Khalil A, Osman MM. Nitric oxide as treatment for an emerging epidemic of obesity-related glomerulopathy. Diabetes Res Clin Pract. 2006;74:207–208
- Wesson DE, Kurtzman NA, Frommer JP. Massive obesity and nephrotic proteinuria with a normal renal biopsy. Nephron. 1985;40:235–237
- Najeeb Q, Masood I, Bhaskar N, et al. Effect of BMI and urinary pH on urolithiasis and its composition. Saudi J Kidney Dis Transpl. 2013;24:60–66
- Allard CB, Shuster A, Pinthus JH, et al. Obesometric factors associated with increased skin-to-stone distances in renal stone patients. Can J Urol. 2012;19:6554–6559
- Arrabal-Polo MA, Arrabal-Martin M, Garrido-Gomez J. Calcium renal lithiasis: metabolic diagnosis and medical treatment. Sao Paulo Med J. 2013;131:46–53
- Abu Ghazaleh LA, Budair Z. The relation between stone disease and obesity in Jordan. Saudi J Kidney Dis Transplant. 2013;24:610–614
- Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The association of increasing body mass index and kidney stone disease. J Urol. 2010;183:571–575
- Chen LY, Qiao QH, Zhang SC, Chen YH, Chao GQ, Fang LZ. Metabolic syndrome and gallstone disease. World J Gastroenterol. 2012;18:4215–4220
- Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr. 2004;80:38–44
- West B, Luke A, Durazo-Arvizu RA, Cao G, Shoham D, Kramer H. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741–747
- Jeong IG, Kang T, Bang JK, et al. Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis. 2011;58:383–388
- Ata N, Kucukazman M, Yavuz B, et al. The metabolic syndrome is associated with complicated gallstone disease. Can J Gastroenterol. 2011;25:274–276
- Weikert C, Weikert S, Schulze MB, et al. Presence of gallstones or kidney stones and risk of type 2 diabetes. Am J Epidemiol. 2010;171:447–454
- Kim JM, Lee HL, Moon W, et al. Association between insulin, insulin resistance, and gallstone disease in Korean general population. Korean J Gastroenterol. 2007;50:183–187
- Nervi F, Miquel JF, Alvarez M, et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J Hepatol. 2006;45:299–305
- Chang Y, Sung E, Ryu S, Park YW, Jang YM, Park M. Insulin resistance is associated with gallstones even in non-obese, non-diabetic Korean men. J Korean Med Sci. 2008;23:644–650
- Nakeeb A, Comuzzie AG, Al-Azzawi H, Sonnenberg GE, Kissebah AH, Pitt HA. Insulin resistance causes human gallbladder dysmotility. J Gastrointest Surg. 2006;10:940–948
- Maalouf NM, Sakhaee K, Parks JH, Coe FL, ms-Huet B, Pak CY. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425
- Maalouf NM, Cameron MA, Moe OW, ms-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888
- Ahmed MH, Byrne CD. Potential therapeutic uses for ezetimibe beyond lowering LDL-c to decrease cardiovascular events. Diabetes Obes Metab. 2010;12:958–966
- Ahmed MH. Orlistat and calcium oxalate crystalluria: an association that needs consideration. Ren Fail. 2010;32:1019–1021
- Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235
- Okamoto M, Kohjimoto Y, Iba A, Saji F, Hara I, Shigematsu T. Calcium oxalate crystal deposition in metabolic syndrome model rat kidneys. Int J Urol. 2010;17:996–1003
- Maalouf NM, Cameron MA, Moe OW, ms-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888
- Abate N, Chandalia M, Cabo-Chan Jr AV, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392
- Sakhaee K, ms-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979
- Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. 2010;183:2244–2248
- Daudon M, Lacour B, Jungers P. High prevalence of uric acid calculi in diabetic stone formers. Nephrol Dial Transplant. 2005;20:468–469
- Daudon M, Traxer O, Conort P, Lacour B, Jungers P. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026–2033
- Vollans SR, Sehjal R, Forster JA, Rogawski KM. Emphysematous pyelonephritis in type II diabetes: a case report of an undiagnosed ureteric colic. Cases J. 2008;1:192. doi: 10.1186/1757-1626-1-192
- Chan AC, Rohan MJ, Hamid A, Azam A. Emphysematous pyelonephritis in a diabetic patient with pelvic-ureteric stone. Med J Malaysia. 2007;62:166–167
- Chung SD, Liao CH, Sun HD, Wen WC. Emphysematous pyelonephritis with acute renal failure. Urology. 2008;72:521–522
- Chapman BA, Wilson IR, Frampton CM, et al. Prevalence of gallbladder disease in diabetes mellitus. Dig Dis Sci. 1996;41:2222–2228
- Pagliarulo M, Fornari F, Fraquelli M, et al. Gallstone disease and related risk factors in a large cohort of diabetic patients. Dig Liver Dis. 2004;36:130–134
- Elmehdawi R, Elmajberi S, Behieh A, Elramli A. Prevalence of gall bladder stones among type 2 diabetic patients in Benghazi Libya: a case-control study. Libyan J Med. 2009;4:27–30
- Liu CM, Tung TH, Liu JH, Lee WL, Chou P. A community-based epidemiologic study on gallstone disease among type 2 diabetics in Kinmen, Taiwan. Dig Dis. 2004;22:87–91
- Al-Bayati S, Kodayer S. Gallstones in a group of Iraqi patients with type 2 diabetes mellitus. Saudi Med J. 2012;33:412–417
- Serrano PE, Khuder SA, Fath JJ. Obesity as a risk factor for nosocomial infections in trauma patients. J Am Coll Surg. 2010;211:61–67
- Bochicchio GV, Joshi M, Shih D, Bochicchio K, Tracy K, Scalea TM. Reclassification of urinary tract infections in critically ill trauma patients: a time-dependent analysis. Surg Infect (Larchmt). 2003;4:379–385
- Maurer KJ, Carey MC, Fox JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. 2009;136:425–440
- Ahmed MH, Byrne CD. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD). Drug Discov Today. 2007;12:740–747
- Ahmed MH, Abu EO, Byrne CD. Non-Alcoholic Fatty Liver Disease (NAFLD): new challenge for general practitioners and important burden for health authorities? Prim Care Diabetes. 2010;4:129–137
- Semins MJ, Shore AD, Makary MA, Weiner J, Matlaga BR. The impact of obesity on urinary tract infection risk. Urology. 2012;79:266–269
- Haidinger G, Temml C, Schatzl G, et al. Risk factors for lower urinary tract symptoms in elderly men. For the Prostate Study Group of the Austrian Society of Urology. Eur Urol. 2000;37:413–420
- Magan AA, Khalil AA, Ahmed MH. Terlipressin and hepatorenal syndrome: what is important for nephrologists and hepatologists. World J Gastroenterol. 2010;16:5139–5147
- Seager CM, Srinivas TR, Flechner SM. Development of nephrolithiasis in a renal transplant patient during treatment with Cinacalcet. Ann Transplant. 2013;18:31–35
- Badalamenti S, DeFazio C, Castelnovo C, et al. High prevalence of silent gallstone disease in dialysis patients. Nephron. 1994;66(2):225–227

