Abstract
Objective: We evaluated the potential protective effect of hydrogen sulfide (H2S) against GEN-induced nephrotoxicity in rats. Materials and methods: Twenty-four rats were randomly divided into four groups, each consisting of six animals as follows: (1) the rats were control, (2) intraperitoneally injected with GEN 14 consecutive days (100 mg/kg/day), (3) treated with GEN plus %0.9 saline intraperitoneally for 14 days and (4) treated with GEN plus sodium hydrogen sulfide (NaHS)-exogenous H2S donor (56 µmol/kg/day) for 14 days. After 15 days, rats were sacrificed and their kidneys were taken and blood analysis was performed. Twenty-four hours urine collections were obtained in standard metabolic cages a day before the rats were sacrificed. Tubular necrosis and interstitial fibrosis scoring were determined histopathologically in a part of kidneys; nitric oxide (NO), malondialdehyde (MDA) and reduced glutathione (GSH) levels were determined in the other part of kidneys. Statistical analyses were made by the chi-squared test and one-way analysis of variance. Results: Serum urea and creatinine levels were significantly higher in rats treated with GEN alone, than the rats in control and GEN + NaHS groups. The GSH levels in renal tissue of only GEN-treated rats were significantly lower than those in control group, and administration of NaHS to GEN-treated rats significantly increased the level of GSH. The group that was given GEN and NaHS had significantly lower MDA and NO levels in kidney cortex tissue than those that was given GEN alone. In rats treated with GEN + NaHS, despite the presence of mild tubular degeneration and tubular necrosis are less severe, and glomeruli maintained a better morphology when compared with GEN group. Discussion: We can say that H2S prevent kidney damage with antioxidant and anti-inflammatory effect.
Introduction
The kidney is a vital organ, which plays an essential role in health, disease, and overall development and growth. The main function of kidney is to maintain total body fluid volume, its composition and acid–base balance. A number of environmental variables including certain drugs influence these functions.Citation1–3 Gentamicin (GEN) derived from Gram-positive bacteria called Micromonospora purpurea present in soil and water having potential in treating aerobic Gram-negative bacteria. Accumulation of gentamicin in proximal renal tubules may cause nephrotoxicity which leads to brush border network damage.Citation4 The nephrotoxicity involves renal free radical production and accumulation, consumption of antioxidant defense mechanisms, glomerular congestion and acute tubular necrosis,Citation5–7 leading to diminished creatinine clearance and renal dysfunction. The pathological mechanisms also involve elevation of endothelin-1, up-regulation of transforming growth factor-beta (TGF-β), significant increase in monocyte/macrophage infiltration into the renal cortex and medulla, augmentation of oxidative stress, and apoptosis and also necrosis.Citation8,Citation9 Moreover, GEN has also been shown to enhance the generation of reactive oxygen species (ROS). Lipid peroxidation (LPO) mediated by ROS has been suggested as a causative agent of cell death in different pathological states including various models of renal diseases.Citation10 Besides their direct damaging effects on tissues, ROS seem to trigger the accumulation of leukocytes in the tissue involved, and thus cause tissue injury indirectly through activated neutrophils. It has been shown that activated neutrophils secrete enzymes such as myeloperoxidase, elastase, and proteases and liberate oxygen radicals. On the other hand, in vivo and in vitro studies have shown that the scavengers of reactive oxygen metabolites are protective in GEN-induced renal failure.Citation11
As previously, an antifibrotic, antiflammatory and antioxidative agent as hydrogen sulfide (H2S) was described. For decades, hydrogen sulfide (H2S) has been known as a toxic gas, and, together with nitric oxide (NO) and carbon monoxide (CO), it is currently recognized as an endogenous gaseous physiological molecule.Citation12 H2S is synthesized from cysteine by two pyridoxal-5′-phosphate dependent enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), and a pyridoxal-5′-phosphate-independent enzyme, 3-mercaptpyruvate sulfurtransferase (3-MST), in most mammalian tissues, including the kidney.Citation13,Citation14 Progression of fibrosis is associated with oxidative stress, inflammatory responses, vascular tone and intracellular signaling pathways. Recent studies in human and animal have demonstrated involvement of H2S in those factors in various diseases, including atherosclerosis, ischemia and reperfusion (I/R) injury, hypertension and end-stage renal disease (ESRD).Citation13–15 In a previous study, H2S supplementation was associated with the suppressions of oxidative stress, inflammation and nitrosative stress.Citation16
Because of these effects of H2S, in this study we investigated the role of H2S in renal damage with GEN. We evaluated the antifibrotic anti-inflammatory and antioxidative effects of H2S in rat kidneys. The nephroprotective effect of i H2S is previously established in many studies, however, to our knowledge this is the first study in literature concerning the protective role of hydrogen sulfide against GEN nephrotoxicity.
Materials and methods
Drugs and animals
Male Wistar albino rats (200–250 g) were housed in clean plastic cages in a temperature and humidity-controlled facility with a constant 12 h light/dark cycle with free access to food and water. The use of animals and the experimental protocol were approved by the Institutional Animal Care and Use Committee and animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals of Research Council. Gentamicin (GEN) was purchased from Bilim Pharmaceuticals. GEN was dissolved in saline and injected intraperitoneally. Like previous study, sodium hydrogen sulfide (NaHS)-exogenous donor of H2S (Merck, Schuchardt, OHG, Hohenbrunn, Germany), was administered intraperitoneally 56 µmol/kg/day for 14 days.Citation17 NaHS was freshly dissolved with 0.9% saline.
Treatment and experimental design
After a quarantine period of 7 days, 32 rats were randomly divided into four equal groups, each consisting of six animals as follows: (1) controls, (2) intraperitoneally injected with GEN for 14 consecutive days (100 mg/kg/day), (3) treated with GEN plus %0.9 saline intraperitoneally for 14 days and (4) treated with GEN plus NaHS (intraperitoneally 56 µmol/kg/day) for 14 days. Rats were treated for 14 days. NaHS was administered immediately after injection of GEN. After 15 days, rats were killed and their kidneys were taken, and blood analysis was performed. Tubular necrosis and interstitial fibrosis scores were determined histopathologically in a part of kidneys; NO, malondialdehyde (MDA), and reduced glutathione (GSH) levels were determined in the other part of kidneys. Urea, creatinine, sodium (Na) and potassium (K) levels were investigated in a blood analysis.
Biochemical assays
Twenty-four hours after the administration of last doses of NaHS, on 15th day, rats were anesthetized by intraperitoneal injection of ketamine and sacrificed. Renal cortical tissues were separated into two parts for biochemical analysis and light microscopic examination. Blood samples were also taken by cardiac puncture to assess the serum levels of urea and creatinine concentrations. In frozen tissues biochemically malondialdehyde (MDA), end product of lipid peroxidation, reduced glutathione (GSH), non-enzymatic antioxidant, and total nitrite, a stable product of nitric oxide (NO), were evaluated as a means of oxidative stress. Renal impairment was assessed by serum urea and creatinine levels, as well as by the kidney histology. Serum urea, creatinine, Na and K levels were determined with an autoanalyzer (Syncron LX20, Dublin, Ireland) by using commercial Becman Coulter diagnostic kits. Kidney tissue (300 mg) was homogenized in ice-cold tamponade containing 150 mM KCL for determination of MDA. MDA levels were assayed for products of lipid peroxidation. MDA referred to as thiobarbituric acid reactive substance, was measured with thiobarbituric acid at 532 nm using a spectrofluorometer, as described previously.Citation18 GSH was determined by the spectrophotometric method, which was based on the use of Ellman’s reagent.Citation19 Total nitrite (NOx) was quantified by the Griess reactionCitation20 after incubating the supernatant with Escherichia coli nitrate reductase to convert NO3 to NO2. Griess reagent (1 ml 1% sulfanilamide, 0.1% naphthyl-ethylenediamine hydrochloride and 2.5% phosphoric acid; Sigma Chemical Co., St. Louis, MO) was then added to 1 ml of supernatant. The absorbance was read at 545 nm after a 30-min incubation. The absorbance was compared with the standard graph of NaNO2, obtained from the reduction of NaNO3 (1–100 mmol/l). The accuracy of the assay was checked in two ways; the inter- and intraassay coefficients of variation were 7.52% and 4.61%, respectively. To check conversion of nitrate to nitrite (recovery rate), known amounts of nitrate were added to control plasma samples; these samples were deproteinized and reduced as above.
Histopathological examinations
Histopathological evaluation of the kidney tissues was done. Paraffin embedded specimens were cut into 6 -μm thickness and stained with Hematoxylin–Eosin stain for light microscopic examination using a conventional protocolCitation21 (Olympus, BH-2, Tokyo, Japan). A semi-quantitative evaluation of renal tissues was accomplished by scoring the degree of severity according to previously published criteria.Citation22 All sections of kidney samples were examined for tubular necrosis. Briefly, minimum of 50 proximal tubules associated with 50 glomeruli were examined for each slide and an average score was obtained. Severity of lesion was graded from 0 to 3 according to the percentage of tubular involvement. Slides were examined and assigned for severity of changes using scores on scale in which (0) denotes no change; grade (1) changes affecting <25% tubular damage (mild); grade (2) changes affecting 25–50% of tubules (moderate); grade (3) changes affecting >50% of tubules (severe). Histopathological evaluation was performed on left kidney tissues. Paraffin-embedded specimens were cut into 5-mm thick sections and stained with hematoxylin and eosin, and Masson’s trichrome for examination under the light microscope (BH-2; Olympus, Tokyo, Japan). To evaluate leukocyte infiltration, the widening of interstitial spaces with focal leukocyte infiltration was assessed in five randomly chosen sections prepared from each kidney sample. For each section, the average number of leukocytes per 0.28 mm2 was calculated from these leukocyte-infiltrated foci using a high-power microscopic field (*400). To estimate the grade of interstitial fibrosis, the interstitial area that was stained green with Masson’s trichrome was evaluated as a percentage of the total examined area in five randomly chosen sections prepared from each kidney sample using an image analyzer (Leica; Leica Micros Imaging Solutions, Cambridge, UK). For each section, interstitial space widening with focal leukocyte infiltration and interstitial fibrosis was assessed in high-power fields (*400) to quantify the results. The Banff classification of kidney pathology was used for scoring the degree of mononuclear cell infiltration and interstitial fibrosis. The score was graded from 0 to 3, depending on the severity of histological characteristics.Citation23
Statistical analysis
Results of all groups were shown as mean values ± SD. Statistical analyses of the histopathologic evaluation of the groups were carried out by the chi-squared test and biochemical data were analyzed by the one-way analysis of variance (ANOVA). The significance between two groups was determined by the Dunnett’s multiple comparison test, and p < 0.05 was accepted as statistically significant value.
Results
No deaths or remarkable signs of external toxicity were observed in the groups of rats given GEN either alone or combination with PE. The biochemical and histopathological results were similar for the control and PE groups, and we decided to consider them without distinction and report only the control group.
Urinary volume
The 24-h urine volume in group GEN was significantly higher than in control group (p < 0.01), indicating the presence of GEN-induced polyuria, whereas in group GEN-NaHS, it was not different from that in control group, pointing out the protective role of NaHS against acute tubular necrosis ().
Table 1. Effects of GEN alone and its combination with H2S on plasma urea, creatinine, Na, K and 24-h urine volume levels in rats.
Biochemical variables in plasma and tissue
Na+ and K+ concentrations among the four groups were similar. Serum urea and creatinine levels were significantly higher in rats treated with GEN alone than the rats in control and GEN + NaHS groups (p < 0.01). Administration of NaHS to the GEN-treated rats caused decrease in serum urea and creatinine levels (). The GSH levels in renal tissue of only GEN-treated rats were significantly lower than those in control group (p < 0.05), and administration of NaHS to GEN-treated rats significantly increased the level of GSH (p < 0.05) ( and ).
Figure 1. (a) Normal tubulus and glomerules in kidney cortex H&E × 100 (control group). (b) Severe tubular necrosis, tubular degeneration and epithelial vacuolization in the proximal tubules ×H&E 100 (GEN-treated group). (c) Moderate tubular necrosis, tubular degeneration and epithelial vacuolization in the proximal tubules H&E × 200 (GEN + vehicle-treated group). (d) Mild epithelial granulovacuolization in the proximal tubules and normal glomerules H&E × 100 (GEN + NaHS-treated group).
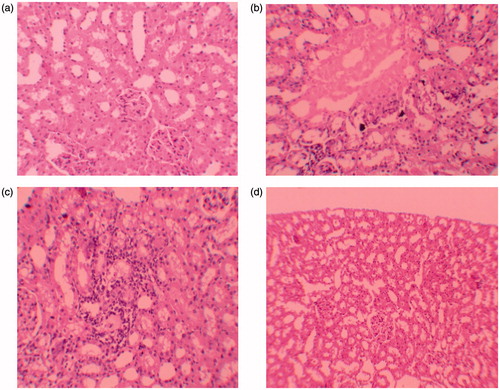
Table 2. Effects of H2S on rat kidney NO, MDA and GSH levels.
The group gave GEN and NaHS had significantly lower MDA levels in kidney cortex tissue than those given GEN alone. There was high level of NO in GEN-treated group; however, NO levels in group treated with GEN + NaHS were significantly lower than GEN-treated group ( and ).
Histopathologic examinations results
Histopathologic examination of kidney showed that there were no pathologic findings in control group (). In rats treated with GEN and GEN + vehicle, there were mild and severe tubular necrosis, tubular degeneration, and epithelial vacuolization in the proximal tubules, and parietal cell hyperplasia compared to control group (). In rats treated with GEN + NaHS, despite the presence of mild tubular degeneration and epithelial vacuolization in the proximal tubules, parietal cell hyperplasia, and tubular necrosis are less severe, and glomeruli maintained a better morphology when compared with GEN group (). These changes are summarized in . After staining with Masson trichrome, no statistical difference was found between groups in kidney fibrosis scores (Table 3 and ).
Figure 2. (a) Severe mononuclear leukocyte infiltration in the cortex of GEN-treated group. (hematoxylin & eosin, ×400). (b) Leukocyte infiltration was reduced in the NaHS-treated group (hematoxylin & eosin, ×400).
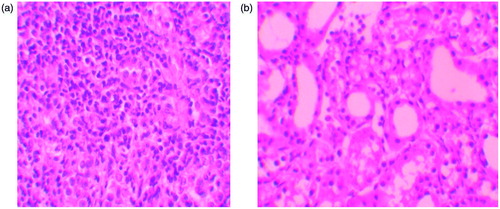
Figure 3. (a) Normal kidney morphology in a control group (masson & trichrome ×200). (b) Severe fibrosis was observed in the peritubular interstitium of the GEN-treated group (masson & trichrome ×400). (c) Mild fibrosis was reduced in the NaHS-treated group (masson & trichrome ×400).
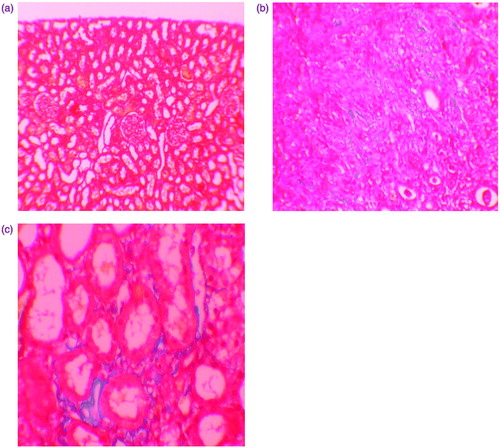
Table 3. Semiquantitative analysis of tubular necrosis, interstitiel fibrosis, leukocte infiltration in control, GEN, GEN + Ve and GEN + NaHS group.
Discussion
The main finding of this study is that hydrogen sulfide can protect renal tubule cells against gentamicin. To the best of our knowledge, this report is the first to show that H2S prevent functional, histological kidney injury by gentamicin. These effects are caused by the antioxidative, anti-inflammatory and anti-apoptotic activities of hydrogen sulfide.
Gentamicin was considered to be an important drug against life-threatening infections, although the mechanism for the nephrotoxic adverse effects is still not very well elucidated.Citation24 Some studies reported that gentamicin-induced reactive oxygen species, which has a major role in the mechanism of nephrotoxicity of gentamicin.Citation25 Gentamicin is still one of the main antibiotics in use against Gram-negative bacteria. Therefore, we are still concerned about the strategies to ameliorate its nephrotoxicity.
The understanding of aminoglycoside nephrotoxicity is clinically important, such as nephrotoxicity is typically associated with non-oliguric acute renal failure, that is, azotemia in the presence of 1–2 l/day urine output. In present study, the 24-h urine volume in the GEN group was significantly higher than in the control group, indicating the presence of GEN-induced polyuria, whereas in GEN + NaHS group, it was not different from that of the control group, suggesting the protective role of H2S against acute tubular necrosis. Also, increased serum urea and creatinine levels in GEN-treated rats reflect the renal damage. In contrast to previous studies, serum K+ levels were similar between group GEN-induced nephrotoxicity and control group. Previous studies show that K+ levels were decreased in rats with GEN-induced nephrotoxicity. It is well documented that GEN nephrotoxicity in experimental animals causes acute renal failure and reduction in serum K+ levels. These results could be attributed to the fact that GEN-induced oxidative stress can promote the formation of a variety of vasoactive mediators that can affect renal function directly by causing renal vasoconstriction or decreasing the glomerular capillary ultrafiltration coefficient and thus reducing glomerular filtration rate (GFR). ROS generated by GEN may also impair the expression of endothelial nitric oxide synthase (eNOS), whereas may scavenge nitric oxide (NO), thereby reducing the amount of the endogenous vasodilator in the vasculature.Citation26,Citation27 Alternatively, the GEN-induced tubular necrosis could lead to a decrease in the number of functioning nephrons, with a subsequent decline in GFR. This effect may trigger multiple adaptive processes in the hyper-functioning remaining nephrons, most notably the alteration in a variety of tubular transport functions including augmented rates of Na+ reabsorption or K+ secretion. The present biochemical finding is confirmed by histopathological examination of GEN-intoxicated kidneys that revealed diffused renal tubular necrosis, associated with periglomerular and perivascular inflammatory cells infiltration, as well as atrophy in the tuft of the glomeruli with thickening in the Bowman’s capsule.Citation2,Citation26–28
Endogenous H2S has been proposed as a novel cytoprotective mediator,Citation29 and there is growing evidence of direct and indirect antioxidant effects of H2S. In cell culture experiments, H2S/HS− generated from NaHS has been shown to “scavenge” detrimental pro-inflammatory oxidants, such as H2O2,Citation30 ClO−,Citation31 superoxide,Citation32 ONOO−33 and NO,Citation32 and also can inhibit cell death induced by these mediators as well as prevent oxidative modification of intracellular proteinsCitation31,Citation33 and LDL (low-density lipoprotein).Citation34 In neuronal cells, NaHS inhibited cell death induced by β-amyloid, mediated at least in part via antioxidant effectsCitation35 and up-regulating intracellular glutathione synthesis through increasing cysteine uptake and elevating γ-glutamylcysteine synthetase activity.Citation36 NaHS is also reported to degrade lipid peroxides,Citation35 inhibit the expression and activity of NADPH oxidaseCitation37 and up-regulate thioredoxin-1 expression in vascular endothelial cells.Citation38 Increased hepatic GSH synthesis and decreased lipid peroxidation are also observed with NaHS treatment in a murine hepatic ischemia/reperfusion injury model.Citation39
Increased lipid peroxidation (LPO) has been reported in renal cortexes by the induction of excessive ROS in renal ischemic reperfusion.Citation40 MDA is the product in the LPO process and is widely used as a reliable marker of tissue damage. In the present study, we found increased MDA levels in GEN and GEN + Ve group, and also as protective effect of H2S lower MDA levels in group determined by GEN + NaHS. The GSH antioxidant system is considered the most notable cellular protective mechanism. GSH has a very important role in protecting against oxygen free radical damage by providing reducing equivalents for several enzymes, as well as scavenging hydroxyl radicals and singlet oxygen. Its depletion is a common consequence of increased formation of ROSCitation41 like GEN-induced nephrotoxicity. In group given GEN + NaHS, we found increased GSH levels. However, our study shown that H2S effect NO levels protectively in similar to some previous studies with different antioxidant agents.Citation42,Citation43 H2S can inhibit NO production and NF-kappa B activation in LPS-stimulated macrophages through a mechanism that involves the action of HO-1/CO.Citation44 Because of that, in our study we found decreased NO levels in GEN + NaHS group compared to UUO group. These findings strongly indicate that H2S is important in protecting the kidney from GEN-induced injury through improvement in oxidant status.
In conclusion, the results reported here indicate that H2S exerts a preventative effect on GEN-induced kidney damage in rats by reducing oxidative stress. One limitation in our study was that the underlying molecular mechanisms that are responsible for the positive effects of NaHS are yet to be determined. We therefore propose that H2S supplementation therapy can be used for kidney protection in patients with GEN-induced nephrotoxicity. However, further animal and clinical studies are needed to confirm our suggestion.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Mahmood I, Waters DH. A comparative study of uranyl nitrate and cisplatin induced renal failure in rat. Eur J Drug Metab Pharmacol. 1994;19:327–336
- Otunctemur A, Ozbek E, Cekmen M, et al. Protective effect of montelukast which is cysteinyl-leukotriene receptor antagonist on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Ren Fail. 2013;35(3):403–410
- Fatima S, Yusufi ANK, Mahmood R. Effect of cisplatin on renal brush border membrane enzymes and phosphate transport. Hum Exp Toxicol. 2004;23:547–554
- Whiting PH, Brown PAJ. The relationship between enzymuria and kidney enzyme activities in experimental gentamicin nephrotoxicity. Ren Fail. 1996;18(6):899–909
- Hur E, Garip A, Camyar A. The effects of vitamin D on gentamicin-induced acute kidney injury in experimental rat model. Int J Endocrinol 2013;2013:313528. doi: org/10.1155/2013/313528
- Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM. Role of myofibroblasts, macrophages, transforming growth factor-β endothelin, angiotensin-II, and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol. 2002;15(6):633–642
- Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009;32(1):61–67
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10(2):248–253
- Bledsoe G, Crickman S, Mao J, et al. Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transpl. 2006;21(3):624–633
- Cekmen M, Otunctemur A, Ozbek E, et al. Pomegranate extract attenuates gentamicin-induced nephrotoxicity in rats by reducing oxidative stress. Ren Fail. 2013;35(2):268–274
- Pedraza-Chaverrıí J, Maldonado PD, Medina-Campos ON, et al. Garlic ameliorates gentamicin nephrotoxicity: relation to antioxidant enzymes. Free Radic Biol Med. 2000;29:602–611
- Banerjee R. Hydrogen sulfide: redox metabolism and signaling. Antioxid Redox Signal. 2011;15:339–341
- Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271
- Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2012;41:113–121
- Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935
- Bracht H, Scheuerle A, Gröger M, et al. Effects of intravenous sulfide during resuscitated porcine hemorrhagic shock. Crit Care Med. 2012;40:2157–2167
- Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27
- Villegas I, Martín AR, Toma W, et al. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor c, protects against gastric ischemia–reperfusion damage in rats: role of oxygen free radicals generation. Eur J Pharmacol. 2004;505:195–203
- Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence sample preservation and storage. Clin Chem. 1993;39:2522–2528
- Beutler E. Glutathione in Red Blood Cell Metabolism. A Manual of Biochemical Methods. New York: Grune and Stratton; 1975:112–114
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500
- Allen CT. Laboratory methods in histochemistry. In: Prophet EB, Mills B, Arrington JB, Sobin LH, eds. American Registry of Pathology. 1st ed. Washington, DC: Armed Forces Institute of Pathology; 1992:53
- Kinugasa F, Noto T, Matsuoka H, et al. Prevention of renal interstitial fibrosis via histone deacetylase inhibition in rats with unilateral ureteral obstruction. Transplant Immunol. 2010;23:18–23
- Ali BH. Gentamicin nephrotoxicity in humans and animals: some recent research. Gen Pharmacol. 1995;26(7):1477–1487
- Jabari M, Rostami Z, Jenabi A, et al. Simvastatin ameliorates gentamicin-induced renal injury in rats. Saudi J Kidney Dis Transpl. 2011;22(6):1181–1186
- Silan C, Uzun O, Comunoglu NU, Gokcen S, Bedirhan S, Cengiz M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull. 2007;30:79–83
- Cronin RE, Thompson JR. Role of potassium in the pathogenesis of acute renal failure. Miner Electrolyte Metab. 1991;17:100–105
- Mohamed Said M. The protective effect of eugenol against gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Fund Clin Pharmacol. 2011;25:708–716
- Whiteman M, Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J Cell Mol Med. 2009;13:488–507
- Muzaffar S, Shukla N, Bond M, et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–528
- Whiteman M, Cheung NS, Zhu YZ, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun. 2005;326:794–798
- Ali MY, Ping CY, Mok YY, et al. Regulation of vascular nitric oxide in vitro and in vivo: a new role for endogenous hydrogen sulphide? Br J Pharmacol. 2006;149:625–634
- Whiteman M, Armstrong JS, Chu SH, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–768
- Muellner MK, Schreier SM, Laggner H, et al. Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL. Biochem J. 2009;420:277–281
- Liu YY, Bian JS. Hydrogen sulfide protects amyloid-β induced cell toxicity in microglia. J Alzheimers Dis. 2010;22:1189–1200
- Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670
- Muzaffar S, Jeremy JY, Sparatore A, Del Soldato P, Angelini GD, Shukla N. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol. 2008;155:984–994
- Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids. 2010;39:1161–1169
- Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:801–806
- Yun Y, Duan WG, Chen P, et al. Ischemic postconditioning modified renal oxidative stress and lipid peroxidation caused by ischemic reperfusion injury in rats. Transplant Proc. 2009;41:3597–3602
- Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009;32:61–67
- Ozbek E, Cekmen M, Ilbey YÖ, et al. Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-kB pathways. Ren Fail. 2009;31:382–392
- Karadeniz A, Yildirim A, Simsek N, et al. Spirulina platensis protects against gentamicin-induced nephrotoxicity in rats. Phytother Res. 2008;22:1506–1510
- Oh GS, Pae HO, Lee BS, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappa B via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–119

