Abstract
Background: Tiopronin, a glycine derivative extensively used to treat cystinuria and hepatic cell injury, can give rise to rare complications such as proteinuria and nephrotic syndrome. However, the pathological characteristics of this secondary nephropathy are poorly understood. Here, we report a case of tiopronin-induced nephrotic syndrome. Case presentation: A 65-year-old Chinese man with a history of myasthenia gravis admitted tiopronin for hepatoprotection therapy. After 3 months later, he presented with rapid weight gain, massive peripheral edema, and proteinuria in the nephrotic range. Laboratory findings included serum albumin (20 g/L), total protein (38 g/L), and total cholesterol (11.78 mmol/L). A 24-hour urine protein collection contained 8620 mg. Percutaneous renal biopsy revealed a uniformly thickened glomerular and rigid basement membrane with immunoglobulin G (IgG) and complement C3 deposited along the glomerular capillary wall. Withdrawal of tiopronin-induced proteinuria complete remission and clinical resolution of nephrotic syndrome. Conclusions: Potential risk of kidney injury exists with long-term tiopronin treatment. Membranous nephropathy was a common renal pathologic feature. Proteinuria in the nephrotic range may spontaneously remit after tiopronin withdrawal. Periodic urine analysis and patient follow-up are recommended with tiopronin therapy.
Background
Tiopronin, a glycine derivative with a reduced sulfhydryl, has been extensively used to treat patients with diverse pathophysiological conditions for more than 30 years. It can scavenge toxic metal ions for chelation therapy,Citation1 protect against nephrotoxicity in patients receiving platinum chemotherapy,Citation2 and reduce cystine disulfide bonds and dissolve cystine stones that develop in the kidneys, ureter, and bladder of patients with cystinuria.Citation3 Tiopronin can also potentially neutralize reactive oxygen species (ROS) via its thiol and it may have anti-inflammatory properties against rheumatoid arthritis.Citation4 In Western countries, tiopronin is used to treat pediatric cystinuria,Citation5 and it may be used to protect against hepatotoxic therapeutic agents, especially in aged populations. Some adverse effects have been reported for tiopronin such as cutaneous side effects (pruritus, erythema, and pemphigus), agranulocytosis, obliterating bronchiolitis, and nephrotic syndrome. Here, we report a case of tiopronin-induced nephrotic syndrome.
Case report
A 65-year-old Chinese male was diagnosed in June 2010 with myasthenia gravis on the basis of palpebral symptoms and a positive neostigmine test. He was given pyridostigmine bromide for initial therapy. During the 17 months of therapy, the myasthenia gravis remitted. Two years after the myasthenia gravis diagnosis, the disease had relapsed twice with symptoms of blepharoptosis and heterotropia. An immunosuppressive regimen was suggested for relapse prevention. The patient was given azathioprine (100 mg/day) as well. Fifty days later, this combination therapy was discontinued despite a partial response due to hepatotoxicity (elevated glutamyl transpeptidase: 278 U/L, normal range; 3–49 U/L and glutamic pyruvic transaminase: 128 U/L, normal range; 0–40 U/L). Subsequently, azathioprine was replaced with tiopronin for hepatoprotection. Ninety-seven days later, the patient was referred to our nephrology division and admitted due to proteinuria and hypoalbuminemia without clinical symptoms other than overt edema in the lower extremities.
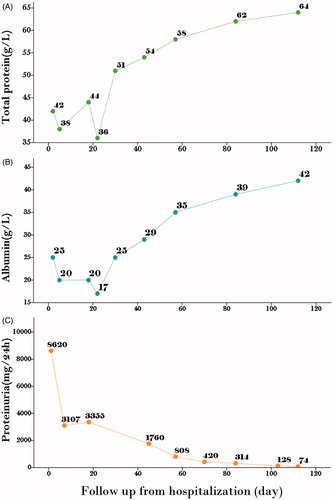
Physical examination revealed mild arterial hypertension and tachycardia (blood pressure = 140/80 mmHg, pulse = 106 beats/min). His temperature was 36.7 °C and he had pitting edema in the bilateral lower extremities with no other abnormalities. Laboratory values at admission are depicted in .
Table 1. Laboratory data at presentation.
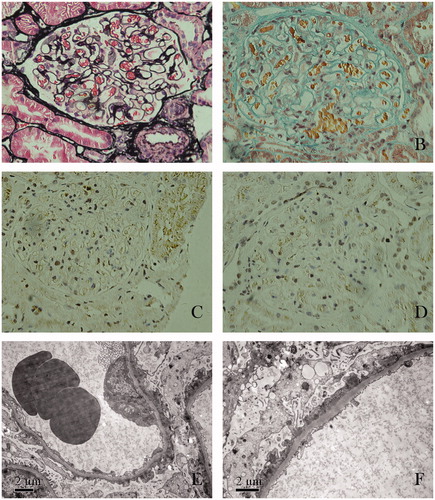
Percutaneous renal biopsy was performed with a 16-gauge needle. Twenty-six glomeruli were counted in the biopsy cylinder. One glomerulus had segmental sclerosis and another had ischemic collapse. Non-sclerotic glomeruli had diffused mild mesangial matrix hyperplasia and hypercellularity. Glomerular basement membranes appeared uniformly thickened and more rigid than normal. The interstitium had a mild focal fibrosis and inflammatory cell infiltration (). Routine immunofluorescent analysis revealed immunoglobulin G (IgG) and complement C3 deposition along the glomerular capillary wall. IgA, IgM, and fibrinogen were negative. Immunohistochemical staining of IgG subclasses revealed that IgG1 and IgG3 deposited along the glomerular capillary wall, whereas IgG2 and IgG4 were negative. Electron microscopy examination revealed that foot process were effaced and immune complexes deposited in the subepithelium. No tubuloreticular inclusions were found in the electron microscopy specimen. These findings were consistent with stage I membranous nephropathy. On the basis of optical microscopic manifestation, we were apt to diagnose atypical membranous nephropathy.
Figure 1. Renal biopsy findings. (A) Glomerular basement membranes appeared uniformly thickened without spikes (stained by periodic methenamine silver, original magnification ×400). (B) Tiny fuchsinophilic grains deposits along the outer aspect of the glomerular basement membranes (stained with Masson’s trichrome, original magnification ×400). (C) Immunoglobulin G subclasses IgG1 deposited along the glomerular capillary wall (stained immunohistochemically, original magnification ×400). (D) Immunoglobulin G subclasses IgG3 deposited along the glomerular capillary wall (stained immunohistochemically, original magnification ×400). (E) Effacement and fusion of epithelial cell foot processes (electron microscopy; original magnification ×8000). (F) Presence of subepithelial immune complex deposits (electron microscopy; original magnification ×10,000).
Tiopronin treatment was stopped and glutathione therapy was initiated at the time of admission. Considering the patient’s age and the potential adverse effect of glucocorticosteroid, cilostazol (50 mg/day) and nadroparin calcium (3800 IU every other day) were administered to prevent thrombotic complications. At the 18th and 70th day of hospitalization, proteinuria was reduced 50% (less than 500 mg/day) and hypoalbuminemia was reversed. At the 103th day of hospitalization, urine protein (128 mg/day) and serum creatinine (54 μmol/L) were in normal range. The patient was in complete remission ().
Discussion
Nephrotic syndrome induced by tiopronin was first reported by Reveillaud et al. in 1978.Citation6 Until now, only 29 clinical cases have been reported in the literatureCitation6–18 (). Most cases occurred in children after treatment of cystinuria. Proteinuria or nephrotic syndrome often occurred 1 to 2 years after tiopronin administration. Rizzoni and co-workers suggested that tiopronin-induced nephrotic syndrome was dose-related and the threshold dose was ∼50 mg/kg/day. A literature review revealed 10 cases diagnosed on the basis of renal pathological characteristics. Reported renal pathological diagnosis included one mesangial proliferative nephritis,Citation9 one membranoproliferative nephritis,Citation9 six membranous nephropathiesCitation9,Citation11,Citation12 and one minimal change nephropathy.Citation16 Previous reports suggest that tiopronin-induced nephropathy required no special therapy such as glucocorticosteroids or immunosuppressive agents. Most patient remissions were due to tiopronin withdrawal.
Table 2. Tiopronin-induced nephrotic syndrome cases reported in the literature.
Our case includes an aged patient who received tiopronin as a hepatoprotection. Nephrotic syndrome occurred 97 days after tiopronin therapy was initiated (600 mg/day; ∼8.82 mg/kg/day). Pediatric patients who received tiopronin for cystinuria treatment have different pathological findings. Renal pathology confirmed membranous nephropathy in our case and this finding was in agreement with published reports. Renal IgG subclass analysis revealed that IgG1 and IgG3 deposited along the glomerular capillary wall. However, IgG2 and IgG4 stains were negative. Electron microscopy examination indicated a fusion of epithelial cell foot processes and the presence of subepithelial immune complex deposits. These, too, were findings that were in agreement with the literature.
Although optical microscopy and electron microscopy revealed that renal pathological manifestations were similar to idiopathic membranous nephropathy, we doubted that this was the situation with this case, and rather, the diagnosis was secondary membranous nephropathy. First, the non-sclerotic glomeruli in the renal biopsy specimen had diffuse mild mesangial matrix hyperplasia and hypercellularity. In idiopathic membranous nephropathy, diffuse global capillary wall thickening in the absence of significant glomerular hypercellularity is a chief finding.Citation19 Idiopathic membranous nephropathy is caused by subepithelial in situ immune complex formation with antibodies from the circulation completing with antigens derived from the capillary wall. Immune complexes formed only at this site could not go against the direction of filtration to reach the mesangium. Secondary forms of membranous nephropathy are usually caused by immune complexes that contain antigens within the circulation. With both antigens and antibodies in the systemic circulation, immune complexes are likely to form that could localize not only in the subepithelial zone but also in the mesangium or subendothelial zone. These immune complexes can stimulate mesangial matrix hyperplasia and hypercellularity.Citation20 Second, IgG subclass analysis revealed that IgG1 and IgG3 deposited along glomerular capillary wall, and IgG2 and IgG4 were negative. Most cases of idiopathic membranous nephropathy have a predominance of IgG4, with less IgG3 and no IgG1 in sub-epithelial immune deposits.Citation21,Citation22 IgG1 and IgG3 are classic complement pathway activators, whereas IgG2 and IgG4 poorly activate complement.Citation23 Finally, the time to complete remission was 3.5 months, a period less than spontaneous remission in idiopathic membranous nephropathy. The appearance of spontaneous remission not induced by immunosuppressive therapy is a well-known characteristic of the idiopathic membranous nephropathy. In a recent study of spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy (Grupo de Estudio de Enfermedades Glomerulares de la Socidad Española de Nefrología [GLOSEN]), Polanco et al. described clinical characteristics of spontaneous remission in 328 patients. The mean time to achieve partial remission and complete remission were 14.7 months (range: 1–66 months) and 38.5 months (range 4–120 months), respectively. Sub-group analysis revealed that the mean time to partial and complete remission were 15.6 months and 41.1 months in a group with baseline proteinuria ranging from 3.5 to 8 g/24 hour, respectively.Citation24
To discover the etiology of secondary membranous nephropathy in our case, we measured Hepatitis B viral marker, tumor marker, antibodies against double-stranded DNA, antibodies against extractable nuclear antigens, and serum immunofixation electrophoresis. Data show that Hepatitis B was not the culprit, and tumor-associated nephritis, lupus nephritis and light chain deposition disease were not mechanisms of nephropathy. Interestingly, our patient had weak positive antinuclear antibody (ANA). ANA subtype analysis showed speckled subtypes and this agreed with the reports in the literature. Ferraccioli et al. reported that six patients who received tiopronin developed nephrotic syndrome. Immunologic analysis revealed a high frequency of ANA positivity by the time nephropathy appeared.Citation9 ANA test positivity correlated better with the appearance of toxicity.Citation25 Similar results were reported by Lindell’s group in 1990. Thirty-two patients with cystinuria were treated with tiopronin, and seven of these patients had positive antinuclear antibodies. In one patient with biopsy-proven membranous glomerulonephritis, antinuclear antibodies and antihistone antibodies were positive.Citation11 In contrast, tiopronin is a D-penicillamine-like drug, which can also induce proteinuria or nephrotic syndrome. Discontinuation of the drug generally results in improvement and steroids may enhance recovery.Citation26 In our case, the magnitude of proteinuria dramatically decreased more than 50% after tiopronin was withdrawn for 1 week. The recovery process was similar to the remission course of D-penicillamine-induced nephropathy.
The pathogenesis of tiopronin-nephropathy has not yet been completely elucidated. Hypothetically, genetic predispositionCitation27 may contribute to its occurrence. Ferraccioli et al. reported a strong association between nephritis due to tiopronin and class I antigens B35-Cw4.Citation28 There were obvious differences between tiopronin-induced and idiopathic membranous nephropathy with respect to IgG subclasses deposited within the glomerulus. Because IgG3 has the strongest affinity for C1q, these findings suggest that tiopronin-induced membranous nephropathy activates the classical pathway more efficiently than idiopathic membranous nephropathy and that the pathogenesis is different between these two diseases.
Conclusion
A potential risk of kidney injury exists with long-term tiopronin therapy. Membranous nephropathy is a common renal pathological finding. Nephrotic-range proteinuria may spontaneously remit after withdrawal of tiopronin. Periodic urine analysis and patient follow-up are warranted during tiopronin therapy.
Declaration of interest
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. The authors declare that they have no competing interests. Z.Z.F. and X.Y. are responsible for patient clinical diagnosis and therapy. They also collected patient follow-up data and drafted the article. S.W.Y. and W.L. performed the renal biopsy and pathological interpretation. J.J.Y. and L.S. prepared the final version of the article. All authors read and approved the final article.
Acknowledgments
We thank Professor Wang for helpful providing electron microscopy photographs.
References
- Fujimoto T, Fuyuta M, Kiyofuji E, et al. Prevention by tiopronin (2-mercaptopropionyl glycine) of methylmercuric chloride-induced teratogenic and fetotoxic effects in mice. Teratology. 1979;20:297–301
- Fetoni AR, Quaranta N, Marchese R, et al. The protective role of tiopronin in cisplatin ototoxicity in Wistar rats. Int J Audiol. 2004;43:465–470
- Chow GK, Streem SB. Medical treatment of cystinuria: Results of contemporary clinical practice. J Urol. 1996;156:1576–1578
- Amor B, Mery C, de Gery A. Tiopronin (N-[2-mercaptopropionyl] glycin) in rheumatoid arthritis. Arthritis Rheum. 1982;25:698–703
- Dello Strologo L, Laurenzi C, Legato A, et al. Cystinuria in children and young adults: Success of monitoring free-cystine urine levels. Pediatr Nephrol. 2007;22:1869–1873
- Reveillaud RJ, Blanc G, Daudon M. Nephrotic syndrome and skin disorders appearing during alpha-mercapto-propionyl-glycine treatment of 2 cases of cystinic lithiasis. J Urol Nephrol (Paris). 1978;84:663–667
- Rizzoni G, Pavanello L, Dussini N, et al. Nephrotic syndrome during treatment with alpha-mercaptopropionylglycine. J Urol. 1979;122:381–382
- Ambanelli U, Manganelli P, Ferraccioli GF. Clinical efficacy and adverse effects of tiopronin in rheumatoid arthritis. Report of a follow-up in 50 patients. Z Rheumatol. 1982;41:235–239
- Ferraccioli GF, Peri F, Nervetti A, et al. Tiopronin-nephropathy: Clinical, pathological, immunological and immunogenetic characteristics. Clin Exp Rheumatol. 1986;4:9–15
- Mordini M, Guidoni G, Maestrini M, et al. Basic treatment of rheumatoid arthritis with tiopronin. A study of 25 cases. Minerva Med. 1989;80:1019–1023
- Lindell A, Denneberg T, Enestrom S, et al. Membranous glomerulonephritis induced by 2-mercaptopropionylglycine (2-MPG). Clin Nephrol. 1990;34:108–115
- Shibasaki T, Murai S, Kodama K, et al. A case of nephrotic syndrome due to alpha-mercaptopropionyl glycine in a patient with familial cystinuria. Nihon Jinzo Gakkai Shi. 1990;32:933–937
- Sany J, Combe B, Verdie-Petibon D, et al. Long-term tolerability of tiopronin (Acadione) in the treatment of rheumatoid arthritis. Apropos of 140 personal cases. Rev Rhum Mal Osteoartic. 1990;57:105–111
- Koeger AC, Palazzo E, De Person JF, et al. Subacute development of nephrotic syndrome caused by tiopronin therapy. A propos of 4 cases. Rev Rhum Ed Fr. 1993;60:78
- Asanuma H, Nakai H, Takeda M, et al. Clinical study on cystinuria in children–the stone management and the prevention of calculi recurrence. Nihon Hinyokika Gakkai Zasshi. 1998;89:758–765
- Lecoules S, Duvic C, Hérody M, et al. Tiopronin-induced nephrotic syndrome with minimal glomerular lesions. Presse Med. 1999;28:273–275
- Alvarez Navascués R, Vidau Argüelles P, Rodríguez Suarez C, et al. Nephrotic syndrome and anasarca status, secondary to treatment with tiopronin in a case of cystinuria. Arch Esp Urol. 2001;54:438–440
- Tasic V, Lozanovski VJ, Ristoska-Bojkovska N, et al. Nephrotic syndrome occurring during tiopronin treatment for cystinuria. Eur J Pediatr. 2011;170:247–249
- Schwartz MM. Membranous Glomerulonephritis. Heptinstall's Pathology of the Kidney. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:205–252
- Beck LH Jr, Salant DJ. Membranous nephropathy: Recent travels and new roads ahead. Kidney Int. 2010;77:765–770
- Doi T, Mayumi M, Kanatsu K, et al. Distribution of IgG subclasses in membranous nephropathy. Clin Exp Immunol. 1984;58:57–62
- Haas M. IgG subclass deposits in glomeruli of lupus and nonlupus membranous nephropathies. Am J Kidney Dis. 1994;23:358–364
- Nangaku M, Couser WG. Mechanisms of immune-deposit formation and the mediation of immune renal injury. Clin Exp Nephrol. 2005;9:183–191
- Polanco N, Gutiérrez E, Covarsí A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704
- Ferraccioli GF, Nervetti A, Mercadanti M, et al. Serum IgA levels and ANA behaviour in rheumatoid patients with and without toxicity to remission-inducing drugs. Clin Exp Rheumatol. 1986;4:217–220
- Koren G. The nephrotoxic potential of drugs and chemicals. Pharmacological basis and clinical relevance. Med Toxicol Adverse Drug Exp. 1989;4:59–72
- Salvarani G, Macchioni P, Rossi F, et al. Nephrotic syndrome induced by tiopronin: Associated with the HLA-DR3 antigen. Arthritis Rheum. 1985;28:595–596
- Ferraccioli G, Peri F, Nervetti A, et al. Toxicity due to remission inducing drugs in rheumatoid arthritis. Assoication with HLA-B35 and Cw4 antigens. J Rheumatol. 1986;13:65–68