Abstract
Background and aims: In order to assess the role played by tubular epithelial cells (TEC) and interstitial vascular endothelial cells (VEC) in interstitial fibrogenesis in human glomerulonephritis, we studied the expression of markers of activated fibroblasts (α-smooth muscle actin (αSMA) and vimentin (Vim)) and of the transforming growth factor β (TGFβ), at the level of these cells. Methods: We studied retrospectively 41 renal biopsies from patients with primary and secondary glomerulonephritis [24 males, 17 females, mean age 45.5 ± 12.9 years]. Immunohistochemistry using monoclonal antibodies (SMA, Vim, TGFβ) was assessed using a semiquantitative score, that was correlated with biological and histological data (quantified using a scoring system in order to assess active-inflammatory and chronic–sclerotic/fibrotic lesions). Results: The presence of SMA and Vim as markers of myofibroblasts was found in TECs and VECs. TEC Vim expression correlated with interstitial Vim expression (r = 0.38; p = 0.008), interstitial infiltrate (r = 0.31; p = 0.027), interstitial fibrosis (R = 0.25; p = 0.042), GFR (r = −0.35; p = 0.016), SMA (r = −0.42; p = 0.015), TGFβ (r = 0.25; p = 0.046), and hemoglobin (r = −0.55; p < 0.001). VEC Vim expression showed indirect correlations with interstitial infiltrate (r = −0.32; p = 0.023) and interstitial fibrosis (r = −0.34; p = 0.017). Conclusion: Our study reflects the complexity of the involvement of VEC and mainly of TEC in fibrosis. The expression of mesenchymal markers at the tubular cell level (especially Vim) correlates with histological interstitial changes, with the decrease of renal function and more strongly with anemia.
Introduction
Chronic kidney disease regardless of its etiology progresses towards end stage renal disease, and the final common pathway in this process seems to be fibrosis.Citation1 An important role is played by tubulo-interstitial fibrosis, which is the strongest morphological predictor of clinical outcome and is most tightly linked to progression of disease, even though the primary disease may be of glomerular origin.Citation2
In a previous study, we have shown that the interstitial histological changes, especially the scores indicating sclerotic/fibrotic lesions, correlate with the presence of interstitial myofibroblasts, with an important role played by TGFβ.Citation3 Thus the main effector cell in this process is the interstitial myofibroblast, being most responsible for interstitial matrix accumulation. Other cells present at this level (tubular epithelial cells (TEC) and vascular endothelial cells (VEC)) are also involved in fibrogenesis.
In the current study performed on human renal biopsies, we have set ourselves to investigate by means of immunohistochemistry, compared with histological and biological data, the possible role of TEC and VEC in interstitial fibrosis.
As shown in experimental studies, these cells are considered possible progenitor cells for myofibroblasts. During the course of kidney fibrosis in mice, about 30% of myofibroblasts are derived via epithelial–mesenchymal transition (EMT) from the TEC of the kidney. In addition, it has been shown that another 35% of myofibroblasts are derived via endothelial–mesenchymal transition (EndMT) from the endothelial cells normally residing within the kidney. The remaining portions are speculated to arise via activation of resident fibroblasts or other mesenchymal cells, such as perivascular smooth muscle cells/pericytes and fibrocytes in the circulation, or are fibroblasts derived from the bone marrow.Citation4,Citation5 Despite these experimental data, the origin of interstitial myofibroblasts remains debatable, because data from in vivo studies is scarce.
In order to assess the role played by TEC and interstitial VEC in human glomerulonephritis, we studied at the level of these cells the expression of mesenchymal markers that are markers of activated fibroblasts (α-smooth muscle actin (αSMA) and vimentin (Vim)) and of the transforming growth factor-β (TGFβ), a growth factor that is involved in this activation.
Materials and methods
Patients
We studied retrospectively the renal biopsies of 41 patients admitted at the Department of Nephrology, Timisoara, with chronic glomerulonephritis (17 females, 24 males; mean age 45.5 ± 12.9 years, range 18–74). Only those cases were included that presented enough paraffin wax embedded in the biopsy material to permit the cutting of additional sections for immunohistochemistry. Cases with fewer than five glomeruli were excluded from the study.Citation6
The patients had either primary (30 cases) or secondary glomerulonephritis (systemic vasculitis, four cases; infectious, three cases; collagenoses, two cases; neoplasia, two cases). The histopathological diagnoses were mesangial proliferative glomerulonephritis (12 cases), mesangiocapillary glomerulonephritis (1 case), membranous nephropathy (5 cases), minimal change disease (5 cases), focal and segmental glomerulosclerosis (15 cases), and crescentic glomerulonephritis (3 cases).
We used as controls renal tissue samples obtained from four patients who underwent nephrectomy for kidney tumors. The samples were obtained from the normal renal tissue. All control patients had normal serum creatinine, eGFR > 60 mL/min, and no proteinuria, at the moment of nephrectomy.
All biopsies were performed after obtaining an informed consent from patients regarding the procedure and the possible use of the obtained material for scientific purposes. The present study has the approval of the local ethical committee.
Histology
Routinely fixed and processed sections of kidney were processed for light microscopy and stained with hematoxylin and eosin (HE), periodic acid-Schiff (PAS), and Gomori's trichrome techniques using routine methods. All stained slides were assessed separately by two pathologists. In order to better quantify the histological lesions, a scoring system adapted by Neumann et al.Citation7 for ANCA-associated vasculitis, based on the standardized scoring system for activity and chronicity developed for lupus nephritis, was employed. At the tubulo-interstitial level, inflammatory lesions (edema, interstitial infiltrate) and sclerotic/fibrotic lesions (interstitial fibrosis, tubular atrophy) were assessed. Tubulo-interstitial lesions were assessed semi-quantitatively: <30% of tubules or interstitial area affected was considered as mild (1 point), 31–60% affected as moderate (2 points), and >60% affected as severe (3 points).Citation7
Immunohistochemistry
The detection of αSMA, Vim, and TGFβ was performed on 4 μm-thick formalin-fixed, paraffin-embedded sections using a horseradish peroxidase-labeled streptavidin–biotin (LSAB2-HRP) method (a system intended for use with primary antibodies for the qualitative identification of antigens in paraffin-embedded tissues).
The primary antibodies used were ready-to-use monoclonal mouse anti-Vim (Vim3B4 antibody, DAKO, Carpinteria, CA); monoclonal mouse anti-smooth muscle actin (clone 1A4, DAKO, Carpinteria, CA); and concentrated monoclonal mouse anti-TGFβ (MCA 797, Serotec, Raleigh, NC).
Sections were first deparaffinized and rehydrated by routine protocol, then incubated with 3% hydrogen peroxide in distilled water for 5 min and afterwards rinsed with distilled water and placed in Tris-buffered saline (TBS) for 5 min. The next step was incubation with a primary antibody, diluted 1/75, for 10–30 min, followed by sequential 10-min incubations with a biotinylated link antibody and peroxidase-labeled streptavidin (both purchased ready-to-use, DAKO, Carpinteria, CA).
The labeling of TGFβ, αSMA, and Vim immunoreactivity, at the level of TEC and VEC, was graded for statistical evaluation using a semi-quantitative intensity scale from 0 to 3, similar to that used by Alexopoulos et al.Citation8 We considered 0 = no labeling (negative), 3 = the most intense labeling, whereas 1 and 2 are labeling of an intermediate degree. The same semi-quantitative intensity scale was used for assessing interstitial Vim staining.
Clinical parameters
In addition to the histological data, we obtained clinical and biological parameters at the time of the biopsy from the patients' files. In all patients, renal function (serum creatinine and glomerular filtration rate (eGFR)), blood pressure, proteinuria (24 h urine specimen), and hemoglobin count were taken. GFR was estimated using the MDRD4 formula.Citation9
Statistical analysis
Data were recorded in a file created in Microsoft Excel, which was organized and managed as a database. Correlations between histological and immunohistochemical parameters were performed using the non-parametric Spearman's rank order test, while correlations among clinical, biological data, and immunohistochemistry were performed using parametric Pearson's test.
Correlation coefficients of linear regression analysis are presented in relation with p values. The significance of the correlation coefficient (r) is as follows: r = 0–0.25 indicates little or no correlation; r = 0.25–0.50 indicates a fair degree of relationship; r = 0.5–0.75 indicates moderate to good correlation; r = 0.75–1 indicates very good to excellent correlation.Citation10 In order to perform these tests, we used WinStat for Microsoft Excel and Epi 3.2.2.
Results
In 24 of the studied cases, we found positive Vim immunostaining at the level of TEC; in 5 of the cases, Vim staining was present either at a proximal (two cases) or at a distal (three cases) tubular level. The mean expression of Vim in TEC was 0.77 ± 0.87 in proximal tubules and 0.71 ± 0.76 in distal tubules. There was, however, no statistically significant difference between the two tubular segments overall.
Vim expression was low or moderate at the level of TEC in our patients; in only one patient (with mesangiocapillary GN), we had to use the degree 3 from the semi-quantitative scale to express a strong Vim staining. Vim expression was positive in patients with focal segmental glomerulosclerosis (nine patients at proximal level and seven patients at distal level), minimal change disease (two patients at proximal and three at distal level), membranous nephropathy (three at proximal and four at distal level), mesangioproliferative GN (five patients at distal and proximal levels), and crescentic GN and mesangiocapillary GN (in both types: one patient at distal and proximal levels) ().
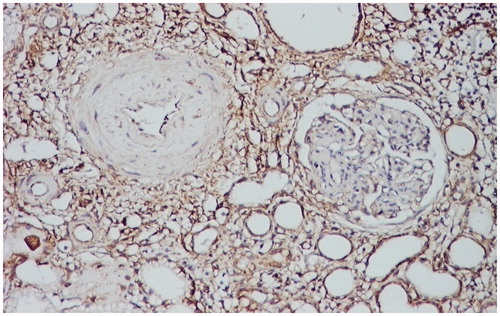
Figure 1. Intense positive vimentin immunostaining at the level of the interstitium, peritubular, and periglomerular. Vimentin stain LSAB2-DAB × 100.
In 13 cases, αSMA immunostaining was present at the level of the TEC. The mean expression of αSMA was 0.55 ± 0.64 in proximal TEC and 0.57 ± 0.69 in the distal ones. This positive αSMA immunostaining occurred in six patients with mesangioproliferative GN, in three patients with focal segmental glomerulosclerosis, in two patients with minimal change disease, in one patient with membranous nephropathy, and in another one with crescentic GN ().
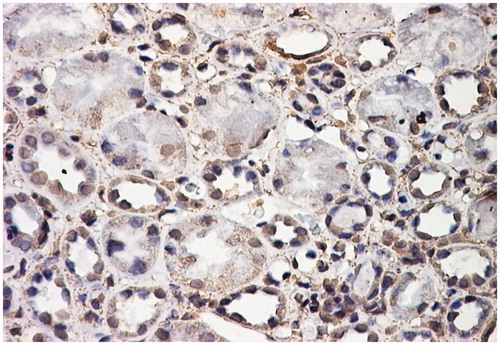
Figure 2. Positive αSMA immunostaining in the cytoplasm and nuclei of tubular epithelial structures. αSMA stain LSAB2-DAB × 200.
TGFβ TEC immunostaining was present in 29 of the studied cases, with no statistically significant difference between the proximal and distal segments of the tubules. The mean expression of TGFβ was 0.97 ± 0.85 in proximal TEC and 1.04 ± 0.86 in distal TEC. Similar to Vim, TGFβ tubular immunostaining was moderate or low, with the exception of two patients in whom we found a strong immunostaining (degree 3). A positive TGFβ immunostaining at TEC level was found in 10 patients with mesangioproliferative GN, in 9 patients with focal segmental glomerulosclerosis, 5 patients with minimal change disease, in 3 patients with membranous nephropathy, in 1 patient with crescentic GN, and in 1 patient with mesangiocapillary GN ().
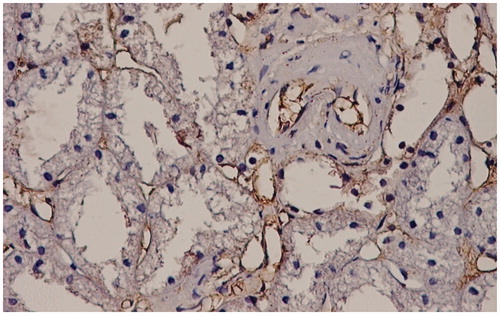
Figure 3. Interstitial capillary and arteriolar endothelium positive for TGFβ stain. TGFβ stain LSAB2-DAB × 200.
At the level of the VEC, Vim staining was present in 27 cases, while TGFβ in 35 cases. The mean expression of Vim was 0.78 ± 0.58 and, for TGFβ, it was 0.92 ± 0.46. At the level of VEC, Vim was positive in 11 patients with mesangioproliferative GN, in 10 patients with focal segmental glomerulosclerosis, in 5 patients with minimal change disease, in 4 patients with membranous nephropathy, in 1 patient with mesangiocapillary GN, and none with crescentic GN. TGFβ was present in 11 patients with mesangioproliferative GN, in 13 patients with focal segmental glomerulosclerosis, in 4 patients with minimal change disease, in 3 patients with membranous nephropathy, 1 patient with mesangiocapillary GN, and in 3 patients with crescentic GN.
In the normal control patients, immunostaining was negative for Vim, αSMA, and TGFβ at the level of TEC and VEC. In these renal samples (from normal controls), we found αSMA in the media of the interstitial vessels.
As mentioned above in the patients with glomerulonephritis, the presence of αSMA and Vim as markers of the myofibroblasts, as well as of TGFβ involved in this process with the formation of active fibroblasts, was found at the level of TEC and VEC. The process shows a great variability in each patient, fact that makes a statistical correlation with biological and histological data difficult; however, some correlations have been found.
First we studied the correlations with tubulo-interstitial histological data assessed by light microscopy. The tubulo-interstitial lesions were studied on standard stains in light microscopy (HE, PAS, and Gomori's trichrome) using the scoring system adapted from Neumann et al. As already mentioned in the Material and methods section, the following tubulo-interstitial lesions were assessed semi-quantitatively: inflammatory lesions (edema and interstitial infiltrate) and sclerotic/fibrotic lesions (interstitial fibrosis and tubular atrophy).
We found a statistically significant small degree of correlation between the scores for Vim staining at the level of the proximal TEC and interstitial infiltrate (r = 0.31, p < 0.05), on one hand, and interstitial fibrosis (r = 0.25, p < 0.05), on the other hand. As shown in , there are no other statistically significant correlations between immunohistochemical parameters at the level of the TEC and interstitial histological scores.
Table 1. Correlation between immunohistochemical staining scores at the level of tubular epithelial cells (TEC) and interstitial histological scores and clinical data (prox, proximal; dist, distal).
When comparing the different immunohistochemical parameters, we found the following correlations: Vim staining at the tubular level (both proximal and distal) correlated with interstitial Vim staining (r = 0.38, p < 0.05) and with TGFβ tubular staining (only for the distal tubules) (r = 0.24, p < 0.05). There was also an indirect correlation between αSMA and Vim at the level of the tubules: distal (r = −0.42, p < 0.05) and proximal (r = −0.30, p < 0.05).
We compared immunohistochemical data in all patients at the tubular level with clinical data (serum creatinine, eGFR, proteinuria, and hemoglobin) and we found that Vim staining at the level of distal TEC correlated statistically significantly with renal function: serum creatinine (r = 0.32, p < 0.05) and eGFR (r = −0.36, p < 0.05). Proteinuria correlated indirectly with the proximal TEC Vim (r = −0.27, p < 0.005) and TGFβ (r = −0.30, p < 0.05) staining.
We found also a moderate indirect correlation between the hemoglobin and the Vim staining at the level of the proximal (r = −0.51, p < 0.001) and distal tubules (r = −0.55, p < 0.001).
Due to the relatively small number of patients with the different histological types of glomerulonephritis, we were not able to find some immunohistochemical expression patterns at the level of TEC. We studied, however, the two subgroups of patients that were better represented: patients with focal segmental glomerulosclerosis (FSGS, 15 patients) and patients with mesangioproliferative glomerulonephritis (MPGN, 12 patients).
In MPGN patients, we found a strong indirect correlation between tubular Vim and renal function: proximal TEC Vim with serum creatinine (r = 0.6, p < 0.05) and with eGFR (r = −0.6, p < 0.05); distal TEC Vim with serum creatinine (r = 0.6, p < 0.05) and with eGFR (r = −0.6, p < 0.05). For TGFβ at the level of distal TEC, we found the same correlation with eGFR (r = −0.51, p < 0.05). Surprisingly in the same group of patients (MPGN), αSMA expression in TEC showed a direct correlation with eGFR both in distal (r = 0.59, p < 0.05) and in proximal tubules (r = 0.52, p < 0.1).
In FSGS patients, we found that proximal tubular Vim expression correlates directly with the score for interstitial fibrosis (r = 0.4, p < 0.1), while proximal tubular αSMA expression correlates indirectly with the scores for interstitial infiltrate (r = −0.62, p < 0.1) and with interstitial fibrosis (r = −0.58, p < 0.1). In this subgroup of patients, we found that TGFβ at the level of proximal TEC correlated indirectly with proteinuria (r = −0.39, p < 0.1).
Regarding VEC, Vim staining at this level correlated indirectly with interstitial infiltrate (r = −0.32, p < 0.05) and interstitial fibrosis (r = −0.34, p < 0.05). Concerning clinical data, we found only an indirect correlation between proteinuria and TGFβ staining at the level of the VEC (r = −0.25, p < 0.05) (). No statistically significant correlations have been found between Vim and TGFβ at VEC level with renal function or anemia.
Table 2. Correlation between immunohistochemical scores for vascular endothelial cells (VEC) staining and interstitial histological scores and clinical data.
Discussions
The results of our study showed at the level of tubules and interstitial vessels a great variability of the expression of the studied immunohistochemical markers (Vim and αSMA), and also of the growth factor TGFβ. Despite this great variability, some facts regarding a certain pattern of staining in these cell types can be discussed.
At the level of TEC, the expression of the mesenchymal markers αSMA and Vim could indicate a process of EMT, a biological process in embryological development.Citation11 In different studies (especially in vitro or in experimental animal models), it has been tried to establish whether EMT also occurs in renal epithelial cells, following kidney injury, and to show that the mesenchymal cells formed could give rise to myofibroblasts which populate the renal interstitium, causing fibrosis within it.Citation12,Citation13
In the cases studied by us, the expression of Vim as the mesenchymal marker at the level of proximal TEC correlated with histological interstitial markers of activity (interstitial infiltrate) and of chronicity (interstitial fibrosis—especially in our FSGS patients). We found also correlations between tubular and interstitial Vim expression. It has already been shown by other authors that in human biopsies of kidneys with fibrotic lesions, Vim is positive at the tubular level.Citation14 Rastaldi et al. have also shown in human biopsies that tubular Vim correlated with the interstitial infiltrate and with interstitial fibrosis, on one hand. In the same study, it was shown that, on the other hand, tubular αSMA was rare, despite the fact that its interstitial expression is a good marker of renal disease progression.Citation15 Our results were consistent with these data, αSMA was present in only 13 cases at the tubular level; moreover, when was assessed quantitatively, it correlated indirectly with Vim expression. We could also observe that especially in the subgroup of FSGS patients, tubular αSMA showed an indirect correlation with interstitial fibrosis, showing thus an opposite pattern to the tubular Vim expression.
TGFβ, the other marker used by us, has the ability to induce the expression of extracellular matrix proteins in mesenchymal cells and to stimulate the production of protease inhibitors that prevent enzymatic breakdown of the ECM. Elevated TGFβ expression in affected organs, and subsequent deregulation of TGFβ functions, correlates with the abnormal connective tissue deposition observed during the onset of fibrotic diseases.Citation16 Therefore, we used TGFβ antibodies in our study to find out if there is an involvement of this growth factor in the activation of TEC. According to Fragiadaki et al., there is no doubt that proximal TEC can undergo EMT in vitro in response to TGFβ-1 and also other inflammatory stimuli.Citation12 The consequence of TGFβ stimulation, due to injury, is an increased expression of Vim in the tubular epithelium.Citation17
In our patients, TGFβ staining correlated with Vim staining at the level of the distal tubules. If we maintain the hypothesis, proven in experimental studies, that TGFβ can promote EMT at the tubular level, then the positive correlation between these two stainings (Vim and TGFβ) can be explained.
The expression of the mesenchymal markers at the tubular level could be correlated with the renal function at the moment of the renal biopsy. In the study performed by Rastaldi et al., tubular Vim correlated with serum creatinine.Citation15 In another study performed by de Matos et al. on 49 kidney transplant recipients, it was shown that a high expression of tubular Vim was associated with a reduction of graft function.Citation18 All these data are consistent with our results concerning the correlation between tubular Vim and renal function (serum creatinine and eGFR) at the moment of renal biopsy. This fact was especially true in the subgroup of patients with MPGN. In this same subgroup of patients, however, αSMA showed, surprisingly, a direct correlation with eGFR.
Urinary proteins from patients with minimal change disease and, especially FSGS, induce in cell cultures the expression of αSMA and Vim.Citation19 In the aforementioned study performed on human renal biopsies by Rastaldi et al., there was a strong correlation between proteinuria and tubular Vim.Citation15 Surprisingly proteinuria showed in our patients an indirect correlation with tubular Vim. Similar to our results, Yonemoto et al. found in patients with diabetic nephropathy that newly acquired Vim (at the mesangial level in their study) decreased in patients with heavy proteinuria.Citation20 Also, in a study performed on patients with congenital nephrotic syndrome of the Finnish type (NPHS1), it was shown that heavy proteinuria did not lead to the transition of the TEC into myofibroblasts, as shown by the expression of tubular Vim and αSMA.Citation21 Moreover, in our patients (especially the FSGS patients), there was also an indirect correlation between tubular TGFβ and proteinuria.
We found an interesting indirect correlation between Vim at the TEC level and hemoglobin. This could be due to the fact that EPO-producing fibroblasts transform into myofibroblasts at the cost of EPO production. This finding could clarify the link between renal fibrosis and anemia.
Studies using in situ hybridization and the transgenic mice approach indicate that EPO is mainly produced by the interstitial fibroblasts in the deep cortex and the outer medulla in the kidney. Asada et al. demonstrate the occurrence in the kidney interstitium of fibroblasts that produce EPO, and can transdifferentiate into scar-producing myofibroblasts, thus losing their EPO-producing activity after kidney injury.Citation22
The fact that in the patients studied by us there was an indirect correlation between tubular Vim and hemoglobin could indicate the fact that EPO-producing fibroblasts have been replaced by myofibroblasts (with Vim expression). It is, however, interesting that for SMA (the other myofibroblast marker), these correlations have not been found.
The few studies performed using human renal biopsies, including the present one, show a correlation of tubular Vim staining with interstitial infiltrate and fibrosis, with renal function and with anemia. Our results regarding TEC could support the hypothesis shown mainly in experimental (in vitro) studies that these cells may acquire markers of mesenchymal cells, a process known as EMT. This possible origin of interstitial myofibroblasts remains debatable.
Humphreys et al. have shown in vivo, but in a mouse model of ureteral obstruction that after injury TEC do not migrate and do not transform into myofibroblasts.Citation23 In another study performed using proximal TEC cultures, it has been shown that shear stress, as it occurs in hyperfiltration, leads to matrix generation and thus fibrogenesis, but inhibits the motility of tubular cells, thus excluding EMT. In contrast, incubation with TGFβ induces cell motility and Vim expression in the cultured tubular cells. The authors conclude that renal fibrosis and EMT could exclude each other.Citation24 This could be a possible explanation of the fact that in our patients with FSGS tubular αSMA correlated indirectly with interstitial fibrosis, or that it correlated directly with eGFR (in patients with MPGN). It remains, however, difficult to explain why in our patients Vim and αSMA showed different patterns of expression and correlations in TEC. It is possible that the two markers are not present at the same time in the different TEC that did undergo a transformation. The fact that we were not able to perform double staining (Vim and αSMA) could be considered a limitation of this study. It is also possible that there are different patterns of expression of these markers in TEC in different histological types of glomerulonephritis, as we have shown for FSGS or MPGN, but the reduced number of cases did not permit the drawing of a pertinent conclusion.
EndoMT, a newly recognized type of cellular transdifferentiation, has emerged as another possible source of tissue myofibroblasts. EndoMT is a complex biological process in which endothelial cells lose their specific markers and acquire a myofibroblastic phenotype and express mesenchymal markers. Similar to EMT, EndoMT can be induced by TGFβ.Citation25 It has been shown in experimental diabetic nephropathy in mice that endothelial–myofibroblast transition occurs and contributes to the early development and progression of renal interstitial fibrosis.Citation26 In the cases studied by us, however, we found that, unlike in TEC, Vim expression in VEC showed an indirect correlation with interstitial histological scores. This surprising fact together with the absence of other correlations between the expression of Vim or TGFβ at the level of VEC with histological and clinico-biological parameters could lead to the conclusion that these cells do not undergo a transition into mesenchymal cells, but the presence of these markers at this level opens the perspective to new studies in this field.
Conclusions
Our study performed in human biopsies reflects the complexity of the involvement of VEC and mainly of TEC in fibrosis. It cannot be stated that TEC are, without doubt, transformed into myofibroblasts during renal injury, but it has been shown that the expression of mesenchymal markers at the tubular cell level (especially Vim) correlates with histological interstitial changes, with the decrease of renal function and more strongly with anemia.
The possible link between renal fibrosis and anemia could be important in developing new treatment strategies that are aimed to target both the renal anemia and the reversibility of fibrosis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Okada H, Strutz F, Danoff TM, Neilson EG. Possible pathogenesis of renal fibrosis. Kidney Int. 1996;49(Suppl. 54):S37–S38
- Barnes JL, Glass WF 2nd. Renal interstitial fibrosis: a critical evaluation of the origin of myofibroblasts. Contrib Nephrol. 2011;169:73–93
- Bob FR, Gluhovschi G, Herman D, et al. Histological, immunohistochemical and biological data in assessing interstitial fibrosis in patients with chronic glomerulonephritis. Acta Histochem. 2008;110(3):196–203
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784
- Kalluri R, Weinberg RA. The basics of epithelial–mesenchimal transition. J Clin Invest. 2009;119(6):1420–1428
- Ponticelli C, Mihatsch MJ, Imbasciati E. Renal biopsy: indications for and interpretation. In: Davison AM, Cameron JS, Grunfeld JP, et al., eds. Oxford Textbook of Clinical Nephrology. 3rd ed. Oxford: Oxford University Press; 2005
- Neumann I, Kain R, Regele H, et al. Histological and clinical predictors of early and late renal outcome in ANCA-associated vasculitis. Nephrol Dial Transplant. 2005;20:96–104
- Alexopoulos E, Stangou M, Papagianni A, Pantzaki A, Papadimitriou M. Factors influencing the course and response to treatment in primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:1348–56
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2005;67:2089–2100
- Dawson B, Trapp RG. Basic and Clinical Biostatistics. New York: McGraw-Hill; 2004
- Eriksson JE, Dechat T, Grin B, et al. Introducing intermediate filaments: from discovery to disease. J Clin Invest. 2009;119(7):1763–1771
- Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis – evidence for and against. Int J Exp Pathol. 2011;92(3):143–150
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–350
- Essawy M, Soylemezoglu O, Muchaneta-Kubara EC, et al. Myo fibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant. 1997;12:43–50
- Rastaldi MP, Ferrario F, Giardino L, et al. Epithelial–mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62(1):137–146
- Verrecchia F, Mauviel A. Transforming growth factor beta and fibrosis. World J Gastroenterol. 2007;13(22):3056–3062
- Kaneyama T, Kobayashi S, Aoyagi D, Ehara T. Tranilast modulates fibrosis, epithelial–mesenchymal transition and peritubular capillary injury in unilateral ureteral obstruction rats. Pathology. 2010;42(6):564–573
- de Matos AC, Câmara NO, Tonato EJ, et al. Vimentin expression and myofibroblast infiltration are early markers of renal dysfunction in kidney transplantation: an early stage of chronic allograft dysfunction? Transplant Proc. 2010;42(9):3482–3488
- Wen Q, Huang Z, Zhou SF, Li XY, Luo N, Yu XQ. Urinary proteins from patients with nephrotic syndrome alters the signalling proteins regulating epithelial–mesenchymal transition. Nephrology (Carlton). 2010;15(1):63–74
- Yonemoto S, Machiguchi T, Nomura K, Minakata T, Nanno M, Yoshida H. Correlations of tissue macrophages and cytoskeletal protein expression with renal fibrosis in patients with diabetes mellitus. Clin Exp Nephrol. 2006;10(3):186–192
- Kuusniemi AM, Lapatto R, Holmberg C, Karikoski R, Rapola J, Jalanko H. Kidneys with heavy proteinuria show fibrosis, inflammation, and oxidative stress, but no tubular phenotypic change. Kidney Int. 2005;68(1):121–132
- Asada N, Takase M, Nakamura J, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest 2011;121(10):3981–3990
- Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97
- Grabias BM, Konstantopoulos K. Epithelial–mesenchymal transition and fibrosis are mutually exclusive responses in shear-activated proximal tubular epithelial cells. FASEB J. 2012;26(10):4131–4141
- Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial–mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179(3):1074–1080
- Li J, Qu X, Bertram JF. Endothelial–myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175(4):1380–1388

