Abstract
Hyperphosphatemia is a risk factor for arterial calcification contributing to the high-cardiovascular mortality in patients with chronic kidney disease (CKD). Ferric citrate hydrate (JTT-751) is being developed as a treatment for hyperphosphatemia with chronic renal failure and has shown a serum phosphorus-lowering effect in CKD patients. In this study, we evaluated the combination effect of JTT-751 with the phosphorus absorption-reducing effect of calcium carbonate and compared phosphorus absorption-reducing efficacy between three phosphate binders including JTT-751. Normal rats were fed a diet containing either 1% calcium carbonate, 1% JTT-751 or 1% JTT-751 with 1% calcium carbonate, for 7 days. Both 1% calcium carbonate and 1% JTT-751 alone reduced urinary phosphorus excretion, and the combined treatment reduced it more than each single-treatment, without clearly influencing calcium or iron-metabolism. Next, normal rats were fed a diet containing either 0.3, 1 and 3% lanthanum carbonate or 2.3% JTT-751, for 7 days. Either 3% lanthanum carbonate or 2.3% JTT-751 reduced urinary phosphorus excretion. Finally, we compared the reduced amount of urinary phosphorus excretion per dose of compound, of which JTT-751 is comparable to that of calcium carbonate and is greater than that of the lanthanum carbonate. In conclusion, JTT-751 showed an additive effect on the phosphorus absorption-reducing effect of calcium carbonate without influencing calcium- and iron-metabolism, and had a phosphorus absorption-reducing efficacy comparable to or greater than other existing phosphate binders.
Introduction
Phosphorus retention is a major harmful complication of chronic kidney disease (CKD), leading to ectopic calcification and significant risk for cardiovascular morbidity and mortality. Secondary hyperparathyroidism, induced by hyperphosphatemia and 1,25-(OH)2 vitamin D3 (1,25-(OH)2D3) deficiency, is accompanied by parathyroid hyperplasia and excessive synthesis and secretion of parathyroid hormone, which results in renal osteodystrophy. Since restriction of intestinal phosphate absorption is necessary to prevent the hyperphosphatemia followed by the progression of serious complications, it becomes necessary for CKD patients with hyperphosphatemia to use phosphate binders. In order to appropriately control the serum phosphorus level, these patients are often treated with calcium-based phosphate binders, frequently combined with 1,25-(OH)2D3. However, long-term use of calcium salts could be involved in the development of hypercalcemia and net calcium retention within the body, potentially inducing ectopic calcification, especially of the blood vessels. Thus, the dose of calcium-based phosphate binders has been limited due to fear of the increase of cardiovascular event and all-cause mortality compared to noncalcium-based phosphate binders. In the last decade, Sevelamer hydrochloride/carbonate, a non-absorbed, calcium-free phosphate binder, was developed, but has been reported to cause a number of serious adverse digestive symptoms. Lanthanum carbonate is also a recently developed metal salt-type phosphate binder, but there are possible concerns about the effects on some organs including bone due to accumulation from long-term treatment.Citation1–3
Ferric ion is the physiological element that has very low, free phosphate in saturated phosphate salt solution, and the phosphate, once bound, is difficult to release. Thus, it had been expected that the iron-containing agents could be the useful phosphate binders.Citation4 Ferric citrate hydrate (JTT-751) is a novel calcium-free phosphate binder, currently being developed as a treatment for hyperphosphatemia in CKD patients. Although ferric citrate is a substance with high versatility used as a food additive and is reported to exert a phosphorus-reducing effect in clinical use,Citation5 JTT-751 was developed as a novel active pharmaceutical ingredient with a larger specific surface area, when compared with conventional ferric citrate, resulting in a beneficial effect on its dissolution rate. In fact, JTT-751 shows an excellent serum phosphorus-reducing effect at relatively low doses without serious side effects in hemodialysis and non-dialysis CKD patients.Citation6–9 From now on, it is necessary to evaluate its utility (long-term efficacy and safety profile, combined therapy with calcium-based phosphate binders, etc) in clinical studies.Citation10
The efficacy of phosphate binders can be assessed by evaluating the reduction of urinary phosphorus excretion in non-dialysis CKD patients,Citation9,Citation11 healthy volunteers,Citation12,Citation13 and normal rats,Citation14,Citation15 which indicates the ability of the phosphate binder to reduce gastrointestinal phosphate absorption. In our previous reports, in normal rats, the effect of JTT-751 to bind with phosphate in the gastrointestinal tract promotes the fecal phosphorus excretion and results in a reduction in the gastrointestinal phosphorus absorption and urinary phosphorus excretion.Citation16
In these two normal rat experiments of the compound's effect on urinary phosphorus excretion, we evaluated the additive effects of JTT-751 to those of calcium carbonate with the combined treatment and compared the efficacy between JTT-751, lanthanum carbonate, and calcium carbonate.
Materials and methods
Materials
Calcium carbonate and lanthanum carbonate were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
General experimental protocol
The experimental protocol was approved by the Experimental Animal Ethical Committee of Japan Tobacco Inc.
Male Sprague-Dawley rats, 6 weeks of age, were purchased from Charles River Japan (Tokyo, Japan) and fed a standard powder chow containing 1.08% phosphorus, 1.06% calcium, 24.7% crude protein and 2.2 IU/g vitamin D3 (CE-2, CLEA Japan, Tokyo, Japan). The rats were housed singly in bracket cages or metabolism cages, and food and water were supplied ad libitum. The monitoring period was set for 3 days from day 13 (13 days before the start of the test compound mixed food administration).
The urine and feces were collected every day during the monitoring period, and the urinary and fecal phosphorus were measured, respectively. The mean urinary and fecal phosphorus excretion per day was calculated. Subsequently, body weights were measured and a blood sample was collected from the tail vein to measure the serum phosphorus, calcium, and creatinine levels at the end of the monitoring period (day 10). In addition, the mean food consumption per day during the monitoring period was measured.
The rats were divided into groups of six rats in Experiment 1 and five rats in Experiment 2; the groups were matched with respect to urinary phosphorus excretion. The mixed food, prepared by mixing the test compound with the CE-2 powder chow using a mixer, was fed to the rat for 7 days. Throughout the administration period, the food consumption was measured, and the urine and feces were collected every day. On day 8 (the end of administration period), the body weights were measured, and blood samples were collected to measure each blood and serum parameters.
Administration groups
In Experiment 1, the rats were divided into four groups (control, 1% calcium carbonate, 1% JTT-751, and 1% calcium carbonate + 1% JTT-751). In Experiment 2, the rats were divided into five groups (control, 0.3% lanthanum carbonate, 1% lanthanum carbonate, 3% lanthanum carbonate and 2.3% JTT-751).
Urine, serum, and feces chemistry about phosphorus, calcium, creatinine, and intact PTH
Collected blood samples were centrifuged at 4 °C to obtain the serum. The phosphorus levels in the urine and serum samples were measured by an enzymatic method using a phosphorus/inorganic phosphorus measurement kit (Kyowa Medex Co., Ltd., Tokyo, Japan) with a biochemistry autoanalyzer (7170, Hitachi High-Technologies Corporation, Tokyo, Japan). Calcium levels in the urine and serum samples were measured by the Ortho-Cresolphthalein Complexone method using a calcium assay kit (Roche Diagnostics K.K., Tokyo, Japan) with a biochemistry autoanalyzer. Creatinine levels in the urine and serum samples were measured by an enzymatic method using a creatinine assay kit (Roche Diagnostics K.K., Tokyo, Japan) with a biochemistry autoanalyzer. Serum intact PTH levels were measured using an intact PTH ELISA assay kit (Immutopics Inc., San Clemente, CA).
The collected feces were dried and then heated at 550 °C for 12 h in a muffle furnace for ashing. Fecal samples were prepared by adding concentrated hydrochloric acid to the ashed feces followed by the extraction of phosphorus. The phosphorus levels in the fecal samples were measured by an enzymatic method using a phosphorus/inorganic phosphorus measurement kit.
Serum iron, blood hemoglobin and reticulocyte (Experiment 1)
The iron levels in the serum samples on day 8 in Experiment 1 were measured by the nitroso-PSAP method using an iron measurement kit (Shino-Test Corporation, Tokyo, Japan) with a biochemistry autoanalyzer.
The hemoglobin and reticulocyte levels in the blood samples on day 8 in Experiment 1 were measured using a hematology analyzer (Siemens Healthcare Diagnostics Inc., Tarrytown, NY).
Estimation of phosphorus absorption-reducing efficacy
The phosphorus absorption-reducing efficacy of the test compound was calculated by dividing the reduced amount of urinary phosphorus excretion by that in the control group with either the amount of the test compound's or element's intake in rats treated with either 1% JTT-751, 1% calcium carbonate (Experiment 1) or 3% lanthanum carbonate (Experiment 2). The amount of the test compound's or element's intake was calculated by multiplying the content of the anhydrous test compound or element in the mixed food by the food consumption.
Statistics
The data are expressed as the mean ± standard deviation (SD). A two-sided test was performed with a significance level of 5%.
In Experiments 1 and 2, Bartlett's test for homoscedasticity was performed to compare each administration group with the control group. If homoscedasticity was found, Dunnett's multiple comparison test was performed, and if heteroscedasticity was found, Steel's multiple comparison test was performed.
Results
Additive effects of JTT-751 to calcium carbonate on phosphorus absorption in normal rats (Experiment 1)
In Experiment 1, no significant differences were observed on the body weights, mean food consumption or creatinine levels in serum and urine in the test compound groups compared with the control group ().
Table 1. Effects of JTT-751 and calcium carbonate on body weight, amounts of food consumption and parameters (Exp. 1).
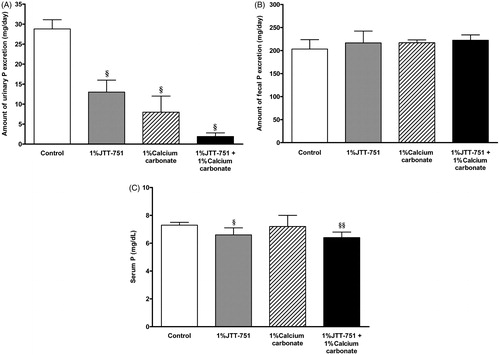
We evaluated whether JTT-751 had additive effects to those of calcium carbonate on phosphate absorption in normal rats. The mean urinary phosphorus excretion was significantly decreased in 1% JTT-751 or 1% calcium carbonate group. The mean urinary phosphorus excretion with the combined treatment was lower than that with each single-treatment (; control, 28.8 ± 2.3 mg/day; 1% JTT-751, 13 ± 3 mg/day; 1% calcium carbonate, 8 ± 4 mg/day; 1% JTT-751 + 1% calcium carbonate, 1.9 ± 0.9 mg/day). The mean fecal phosphorus excretion in each treatment group tended to be increased, but not significantly, compared with that in the control group (). A clear and significant reduction in serum phosphorus level was observed with the combined treatment (). Although there were no significant differences in the serum calcium level in the test compound groups compared with the control group, significant increases in the mean urinary calcium excretion were observed in rats treated with 1% calcium carbonate and with the combined treatment. Serum intact PTH level was significantly decreased in 1% calcium carbonate group. Serum intact PTH level with the combined treatment was lower than that with each single treatment ().
Figure 1. Effects of JTT-751, calcium carbonate and combined treatment on urinary, fecal and serum phosphorus in normal rats. (A) Mean urinary phosphorus excretion in 1% JTT-751 group, 1% calcium carbonate group and combined treatment group compared to control group. Data are the mean ± SD. §p < 0.05 versus control group (Bartlett's test followed by Steel's test). (B) Mean fecal phosphorus excretion. (C) Serum phosphorus level at day 8. §p < 0.05, §§p < 0.01 versus control group (Bartlett's test followed by Steel's test).
There were no significant differences in the serum iron, blood hemoglobin and reticulocyte levels in the test compound groups compared with the control group ().
Effects of lanthanum carbonate on phosphorus absorption in normal rats (Experiment 2)
In Experiment 2, no significant differences were observed in the body weights, mean food consumption or creatinine in serum and urine in the test compound groups compared with the control group ().
Table 2. Effects of JTT-751 and lanthanum carbonate on body weight, amounts of food consumption and parameters (Exp. 2).
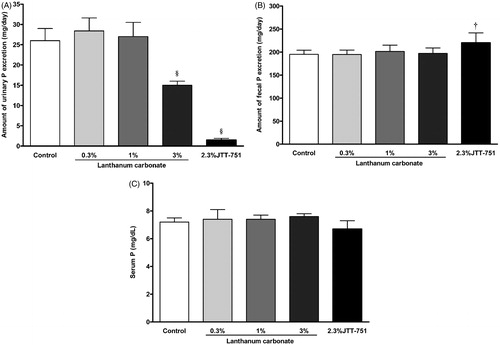
The mean urinary phosphorus excretion was significantly decreased in 3% lanthanum carbonate or 2.3% JTT-751 group (; control, 25.8 ± 3 mg/day; 0.3% lanthanum carbonate, 28.4 ± 3.2 mg/day; 1% lanthanum carbonate, 27.0 ± 3.5 mg/day; 3% lanthanum carbonate, 15 ± 1 mg/day; 2.3% JTT-751, 1.5 ± 0.4 mg/day). The 2.3% JTT-751 treatment significantly increased the mean fecal phosphorus excretion, compared with the control group () and tended to reduce the serum phosphorus level ().
Figure 2. Effects of JTT-751, lanthanum carbonate on urinary, fecal and serum phosphorus in normal rats. (A) Mean urinary phosphorus excretion in lanthanum groups and 2.3% JTT-751 group compared to control group. Data are the mean ± SD. §p < 0.05 versus control group (Bartlett's test followed by Steel's test). (B) Mean fecal phosphorus excretion. †p < 0.05 versus control group (Bartlett's test followed by Dunnett's test). (C) Serum phosphorus level at day 8.
Although there were no significant differences in the serum calcium levels in the test compound groups compared with the control group, significant increases in the mean urinary calcium excretion were observed in rats treated with 2.3% JTT-751 ().
The phosphorus absorption-reducing efficacy of JTT-751, lanthanum carbonate, and calcium carbonate
The phosphorus absorption-reducing efficacy of test compound was calculated by dividing the reduction in urinary phosphorus excretion compared to that in the control group by either the amount of the test compound or element intake in rats. Each element intake was 0.85 mmol Fe/day in the 1% JTT-751 group, 2.26 mmol Ca/day in the 1% calcium carbonate group in Experiment 1 and 2.50 mmol La/day in the 3% lanthanum carbonate group in Experiment 2.
Using the reduction in urinary phosphorus excretion in the 1% JTT-751 group in Experiment 1, the phosphorus absorption-reducing efficacy of JTT-751 was calculated as 83 ± 22 mg phosphorus/g anhydrous compound and 19 ± 5 mg phosphorus/mmol Fe. The phosphorus absorption-reducing efficacy of calcium carbonate also could be calculated as 94 ± 18 mg phosphorus/g anhydrous compound and 10 ± 2 mg phosphorus/mmol Ca using the value of 1% calcium carbonate group in Experiment 1. Using the value of 3% lanthanum carbonate group in Experiment 2, the phosphorus absorption-reducing efficacy of lanthanum carbonate also could be calculated as 19 ± 3 mg phosphorus/g anhydrous compound and 5 ± 1 mg phosphorus/mmol La ().
Table 3. The phosphorus absorption-reducing efficacy of JTT-751, lanthanum carbonate and calcium carbonate.
Discussion
We have already reported that JTT-751 (ferric citrate hydrate) and calcium carbonate showed inhibitory effects on phosphorus absorption in normal rats in vivo. In short, the fecal phosphorus excretion was increased with increasing doses of JTT-751, and the value in the 3% JTT-751 group was significantly higher than that in the control group. One and 3% JTT-751 treatment decreased the urinary phosphorus excretion significantly to ∼50% and ∼5% of the control value. Significant reductions in serum phosphorus and intact PTH level were observed in 3% JTT-751 group. Meanwhile, 3% calcium carbonate showed the same effect on fecal, urinary, and serum phosphorus and intact PTH as 3% JTT-751.Citation16
In this normal rat study, we used urinary phosphorus excretion as a marker of the phosphate binders’ efficacy. It is well known that the efficacy of phosphate binders can be assessed by evaluating urinary phosphorus excretion, which indicates the ability of the phosphate binder to reduce gastrointestinal phosphorus absorption in CKD non-dialysis patients,Citation9,Citation11 healthy human volunteers,Citation12,Citation13 and normal rats;Citation14,Citation15 the greater the amount of phosphate bound in the intestinal lumen and excreted in the feces, the smaller the amount of phosphorus absorbed from the intestine into the body and excreted in the urine.
In clinical use, to avoid hypercalcemia and vascular calcification, the dose of calcium-based phosphate binders is limited. Thus, we evaluated the possibility of a moderate dose of calcium carbonate treatment combined with JTT-751. In Experiment 1, we selected a 1% dose of calcium carbonate and JTT-751 whose reduction of the urinary phosphate excretion was not saturated. In this study design, an additive effect of JTT-751 on the reducing-effect of calcium carbonate in urinary phosphorus excretion was shown. This additive reducing-effect of JTT-751 on phosphorus absorption was supported by the fact that the fecal phosphorus excretion was greater and the serum phosphorus level was lower in the combined treatment group than in each single-treatment. As for the serum intact PTH level, 1% JTT-751 and 1% calcium carbonate reduced it, and the combined treatment reduced it greater than each single treatment.
At the same time, we evaluated the effect of calcium- and iron-metabolism related parameters. As for calcium-metabolism, in our previous study,Citation16 a 3% dose of JTT-751, which showed the same inhibitory effect on phosphorus absorption as a 3% dose of calcium carbonate, slightly increased the serum calcium levels but by less than that of a 3% dose of calcium carbonate. In this study, the serum calcium level was not influenced by the combined treatment with JTT-751. Thus, it is suggested that the amount of calcium load in the combined treatment with JTT-751 could be lower than that in the calcium carbonate single treatment for sufficiently lowering the serum phosphate levels. Although the amount of urinary calcium excretion was slightly more with the combined treatment than that with the calcium carbonate single treatment, it was thought that the phosphate in the intestinal lumen acted as a natural calcium binder, and therefore the phosphate binder's high-effective dosing could increase the amount of free absorbable calcium, as reported with other calcium-free phosphate binders,Citation15,Citation17 2.3% JTT-751 in this Experiment 2 and 3% JTT-751.Citation16 Thus, it was thought that the increase of urinary calcium excretion in the combined treatment was due to the calcium load itself and an increase in free absorbable calcium in the intestinal lumen. As for iron-metabolism in JTT-751 treatment, the serum iron level, blood hemoglobin and reticulocyte were not changed.
We had shown that, under the hyperphosphatemic condition in adenine-induced chronic renal failure (CRF) rats, 35-day-repeated dietary administration of 1% JTT-751 tended to decrease serum phosphorus level, and 3% JTT-751 and 3% calcium carbonate decrease it to below the value in the normal control group. Since 3% JTT-751 and 3% calcium carbonate clearly increase fecal phosphorus excretion in normal rats and apparently decrease serum phosphorus level in CRF rats,Citation16 JTT-751 and calcium carbonate could be the same character about phosphorus absorption-reducing effects regardless of normal or pathophysiological condition. Thus, this combined treatment is expected to show sufficient serum phosphorus-lowering effect without calcium and iron overload in clinical use.
Since the phosphate binders must be used for a long time for hyperphosphatemia in the CKD patients, a smaller dose of the phosphate binder is needed for drug compliance and to avoid any side effects. Thus, in this study, we evaluated the phosphorus absorption-reducing efficacy between JTT-751 and the existing phosphate binders (lanthanum carbonate and calcium carbonate). Although in vitro phosphate-binding studies are one of the possible tools for an estimating binder's phosphate-binding activity in clinical use,Citation14,Citation18 we selected an in vivo evaluation because it is under more physiological conditions, and JTT-751 is a compound which includes citrate, which has a dissolving effect on ferric phosphate especially in in vitro closed conditions. Although we divided it into two experiments by similar protocols due to our capacity of carrying out a single experiment, the amounts of urinary phosphorus excretion in the control groups were almost same between two experiments in this study. Thus, we judged that it was possible to compare the efficacy of three compounds in the separated experiments.
In Experiment 1 of this study, the reduced amount of the mean 24-h urinary phosphorus excretion was 15 mg after dietary administration of 1% JTT-751 (584 mg/kg and 475 mg/kg as anhydrate), calculated approximately as 83 mg phosphorus/g anhydrous JTT-751 as phosphorus absorption-reducing efficacy. One percentage of calcium carbonate showed 94 mg phosphorus/g anhydrous calcium carbonate as phosphorus absorption-reducing efficacy, which was almost the same as JTT-751. The phosphorus absorption-reducing efficacy of Fe in JTT-751 (mg phosphorus/mmol element) is 19 which is greater than that of Ca in calcium carbonate (10 mg/mmol). It was thought that trivalent ferric ion is an element that has very low free phosphate in its saturated phosphate salt solution because it can hardly release bound phosphate.
Lanthanum carbonate has been reported to have its in vivo phosphate-binding capacity in healthy human volunteers,Citation13 described as “reductions in 24-h urinary phosphorus excretion in volunteers receiving a Lanthanum element dose of 3000 mg/day were between 236 and 468 mg/day over the five separate studies”. Therefore, in humans, phosphorus absorption-reducing efficacy is calculated as between 236 and 468 mg phosphorus/an anhydrous lanthanum carbonate dose of 4944 mg/day, almost equal to approximately between 48 and 95 mg phosphorus/g anhydrous lanthanum carbonate. Furthermore, it was also shown that the average reduction in phosphorus excretion was 95 mg/g of elemental lanthanum (equal to approximately 58 mg/g anhydrous lanthanum carbonate) and 26.5 mg/g of calcium carbonate in healthy volunteers.Citation19 In this rat study, the phosphorus absorption-reducing efficacy of lanthanum carbonate was also calculated as 19 mg/g anhydrous compound and that of calcium carbonate was 94 mg/g. Thus, phosphorus absorption-reducing efficacy of lanthanum carbonate and calcium carbonate is in the same level, but it was not completely consistent with the human healthy volunteer studies. In rats fed chow diet containing 1.08% phosphorus, higher efficient dose (g/kg) and higher solubility might be needed for phosphate binders to show effects than in human. In the results of this study, calcium carbonate had an efficacy greater than lanthanum carbonate probably because calcium carbonate may have higher solubility in the intestinal lumen when administered as mixtures in the diet. In fact, calcium carbonate has higher solubility in water than lanthanum carbonate in in vitro study (A Matsuo et al. unpublished observations). Although the direct comparison in the clinical study is necessary to accurately compare the efficacy between several phosphate binders, at least under the same experimental condition in this rat study, JTT-751, on a per dose basis, showed a phosphorus absorption-reducing efficacy greater than lanthanum carbonate and comparable to calcium carbonate.
In conclusion, JTT-751 could show an additive effect on the phosphorus absorption-reducing effect of calcium carbonate without influencing calcium- and iron-metabolism, and have a phosphorus absorption-reducing efficacy comparable to or greater than other existing phosphate binders.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We thank Akira Miyazaki (Japan Tobacco Inc.) for his research coordination over this study.
References
- Hutchison AJ, Smith CP, Brenchley PE. Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol. 2011;7(10):578–589
- Kazama JJ. Oral phosphate binders: history and prospects. Bone. 2009;45(Suppl 1):s8–s12
- Jamal SA, Vandermeer B, Raggi P, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382(9900):1268–1277
- Hergesell O, Ritz E. Phosphate binders on iron basis: a new perspective? Kidney Int Suppl. 1999;73:s42–s45
- Yang WC, Yang CS, Hou CC, Wu TH, Young EW, Hsu CH. An open-label, crossover study of a new phosphate-binding agent in hemodialysis patients: ferric citrate. Nephrol Dial Transplant. 2002;17(2):265–270
- Sinsakul M, Sika M, Koury M, et al. The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract. 2012;121(1-2):c25–c29
- Umanath K, Sika M, Niecestro R, et al. Rationale and study design of a three-period, 58-week trial of ferric citrate as a phosphate binder in patients with ESRD on dialysis. Hemodial Int. 2013;17(1):67–74
- Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y. Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol. 2012;36(5):478–487
- Yokoyama K, Hirakata H, Akiba T, et al. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol. 2014;9:543–552
- Ogata H, Koiwa F, Shishido K, Kinugasa E. Combination therapy with sevelamer hydrochloride and calcium carbonate in Japanese patients with long-term hemodialysis: alternative approach for optimal mineral management. Ther Apher Dial. 2005;9(1):11–15
- Sprague SM, Abboud H, Qiu P, Dauphin M, Zhang P, Finn W. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin J Am Soc Nephrol. 2009;4(1):178–185
- Burke SK, Slatopolsky EA, Goldberg DI. RenaGel, a novel calcium- and aluminium-free phosphate binder, inhibits phosphate absorption in normal volunteers. Nephrol Dial Transplant. 1997;12(8):1640–1644
- Pennick M, Poole L, Dennis K, Smyth M. Lanthanum carbonate reduces urine phosphorus excretion: evidence of high-capacity phosphate binding. Ren Fail. 2012;34(3):263–270
- Rosenbaum DP, Holmes-Farley SR, Mandeville WH, Pitruzzello M, Goldberg DI. Effect of RenaGel, a non-absorbable, cross-linked, polymeric phosphate binder, on urinary phosphorus excretion in rats. Nephrol Dial Transplant. 1997;12(5):961–964
- Damment SJ. Pharmacology of the phosphate binder, lanthanum carbonate. Ren Fail. 2011;33(2):217–224
- Iida A, Kemmochi Y, Kakimoto K, et al. Ferric citrate hydrate, a new phosphate binder, prevents the complications of secondary hyperparathyroidism and vascular calcification. Am J Nephrol. 2013;37:346–358
- Nagano N, Miyata S, Obana S, et al. Renal mineral handling in normal rats treated with sevelamer hydrochloride (Renagel), a noncalcemic phosphate binder. Nephron. 2001;89(3):321–328
- Autissier V, Damment SJ, Henderson RA. Relative in vitro efficacy of the phosphate binders lanthanum carbonate and sevelamer hydrochloride. J Pharm Sci. 2007;96(10):2818–2827
- Daugirdas JT, Finn WF, Emmett M, Chertow GM; Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin Dial. 2011;24(1):41–49

