Abstract
Podocytes are highly differentiated epithelial cells that form interdigitating foot processes with bridging slit diaphragms (SDs) that regulate renal ultrafiltration. Proteinuria is the most common clinical manifestation of glomerular diseases. Losartan is the traditional renin–angiotensin system (RAS). However; the precise mechanisms underlying the beneficial effects of Losartan on podocytes are still unknown. This study tested the hypothesis that podocytes were exposed to Angiotensin II (Ang II) to induce apoptosis and Proteinuria. Losartan directly reduces the rate of apoptotic podocytes induced by Ang II. Our results showed that apoptotic podocytes may be related to the decrease of CD2AP mRNA and protein expressions, Losartan reduced the apoptosis and Proteinuria by stabilizing the CD2AP mRNA and protein expressions. In this study, we explored the role of CD2-associated protein in podocyte apoptosis and Proteinuria induced by Ang II. Our findings provide some possible clues for further exploring the pharmacological targets to the proteinuria.
Introduction
Glomerular disease is one of the main causes of chronic kidney disease (CKD), and either primary or secondary glomerular diseases, the common features were proteinuria occurrence and foot processes fusion or loss. Therefore, podocytes play a key role in the pathogenesis of glomerular disease. Proteinuria stems from injury to podocytes, terminally differentiated cells that reside in the kidney glomeruli, the location of the renal filtration barrier. CD2AP is expressed primarily in podocytes. Given the important role of CD2AP in podocyte apoptosis and Proteinuria, we hypothesized that Ang II may result in podocyte apoptosis and Proteinuria via down-regulation of CD2AP protein expressions, and rescue of CD2AP expression may protect podocytes from undergoing apoptosis by Losartan. This study aims to briefly summarize and discuss our findings regarding the roles of CD2AP molecule in regulation of podocyte apoptosis and Proteinuria induced by Ang II Shed new light on the pathogenesis of proteinuria. And provide more clear theoretical basis for the molecular mechanism of podocyte apoptosis and Proteinuria. This study tested the hypothesis that Losartan reduced the apoptosis and Proteinuria by stabilizing CD2AP mRNA and protein expressions, and protected podocytes. In summary, our results demonstrate.
Materials and methods
Cell culture and treatments
Podocytes were grown in RPMI 1640 medium that contained 10% FBS (Sigma Chemical Co., St. Louis, MO), penicillin (100 U/mL), streptomycin (100 µg/mL), sodium pyruvate (Sigma Chemical Co.), and sodium bicarbonate (Sigma Chemical Co.). Podocytes were grown under “growth permissive” conditions, which involved growing cells at 33 °C in the presence of IFN-γ (Sigma Chemical Co.). For podocytes to acquire a differentiated and quiescent phenotype, cells were grown under “restrictive conditions” at 37 °C in 95% air/5% CO2 without IFN-γ for >14 d. In the studies described below, growth-restricted podocytes were used. The experiment set up the control group, Ang group and Losartan group. Drugs before treatment, The control group was treated by the RPMI DMSO containing 0.02% 1640 medium; Ang II group: Ang II was added to the medium at the concentration of 10−8 mol/L (Sigma Chemical Co.); Losartan group: Cells were incubated with Losartan (10−5 mol/L, Sigma Chemical Co.) and the Ang II (10−8 mol/L). All experiments were performed a minimum of three times.
RT-PCR analysis
The primer and probe design: CD2AP upstream primer sequence: 5′-GCTGGTGGAAAGGTGAACTG-3′; Downstream primer sequence: 5′-CATCTCTGTCTTCCGCCTTC-3′, product size for 192bp. GAPDH upstream primer sequence: 5′-GGTGAAGGTCGGTGTGAACGGAT-3′; Downstream primer sequence: 5′-CCACTTTGCCACTGCAAATGGCAG-3′, product size for 77 bp. Briefly, total RNA was isolated from podocytes, using Trizol reagent (Invitrogen, Shanghai, China) according to the manufacturer's instructions. The integrity of the total RNA was examined by 1% agarose gel electrophoresis, the quantity was determined based on absorbance at 260 nm (A260), and the purity was analyzed based on the absorbance ratio at 260 and 280 nm (A260/280). The cDNA was synthesized from 1 μg of total RNA using AMV reverse transcriptase XL (TaKaRa, Dalian, China). GAPDH served as an internal control. To create real-time PCR standards, CD2AP and GAPDH were amplified using reverse transcriptase-PCR using specific primers. Real-time PCR was performed independently at least three times. After the reaction, the computer automatic analysis and calculation results.
Western blot analysis
Western blot analysis was performed to measure the protein levels of CD2AP protein in cells that were exposed to Ang II in the presence and absence of Losartan. Cells were washed three times with ice-cold PBS and harvested by trypsin digestion at 37 °C for 3 min. Cells were pelleted by centrifugation (1200 rpm for 3 min at 4 °C), washed twice with ice-cold PBS, and then suspended in lysis buffer that contained 1% Triton, 10% glycerol, 20 mmol/L HEPES, 100 mM NaCl, and protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN). After an overnight freeze–thaw cycle, lysates were cleared by centrifugation at 14,000 rpm for 5 min at 4 °C, and protein concentration was determined by BCA Protein Assay Kit (Pierce, Rockford, IL) according to the manufacturer's protocol. Reducing buffer was added to each protein extract, and samples were boiled for 5 min. Reduced protein sample (5 to 10 µg) then was loaded per lane on a 15% SDS–polyacrylamide gel and subsequently transferred to a polyvinylidene difluoride membrane (PerkinElmer Life Sciences, Boston, MA) by electroblotting at 350 mA for 75 min. After blocking for 30 min in 5% nonfat dried milk, membranes were incubated overnight (4 °C) with the following primary antibodies: Anti-CD2AP (Sigma Chemical Co.), anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). After three wash cycles with Tris-buffered saline with 0.1% Tween, membranes were incubated with an anti-mouse alkaline phosphatase-conjugated secondary antibody (Promega, Guangzhou, China) for 1h at room temperature. The specific protein bands were scanned and quantitated using a densitometry in relation to the GAPDH. Western Blotting Detection System (GE Healthcare, Chalfont St. Giles, UK). We repeated each Western blot analysis using protein from three different and separate experiments.
Apoptosis analysis
For examining the effect of glucocorticoid on Ang II-induced podocyte apoptosis, podocytes grown under growth restrictive conditions for >14 d were plated at a density of 7.5 × 104/cm2 and allowed to attach to tissue culture plates for 24 h. Cells then were incubated with medium that contained 10% FBS in the presence or absence of Losartan (Sigma Chemical Co.). 1hour later, Ang II was added to the medium at the concentration of 30 µg/mL. The percentage of apoptotic cells was assessed. Apoptosis was measured by staining with FITC-Annexin V and PI (Sigma Chemical Co.) take 500 μl cell suspension liquid join 5 μl FITC-Annexin V and 5 μl PI (concentration 250 mg/L), avoiding light for 10 min at 4 °C, excitation wavelength for 488 nm, each specimen collected 10,000 cells, and then relevant software analyzed and calculated cell apoptosis rate.
Statistical analyses
Statistical analysis was performed using SPSS 17.0 (Guangzhou, China); statistical significance was evaluated using t-test. p < 0.05 was considered to be statistically significant. All the experiments in our study were performed at least thrice.
Results
RT PCR analysis
CD2AP plays a central role in Ang II-induced apoptosis, but the role of CD2AP is not well understood in podocyte apoptosis. To elucidate the mechanisms of Ang II-induced podocytes apoptosis and the potential effects of Losartan on specific apoptotic ways, we performed Real-Time PCR to measure the CD2AP mRNA expression. Real-Time PCR showed that In the control, Ang II and Losartan group, CD2AP mRNA expression had no changes at 8h (p > 0.05), incubation of the podocytes with Ang II markedly decreased CD2AP mRNA levels at 24 and 48 h (p < 0.05). In contrast, Losartan completely prevented the mRNA decrease in CD2AP induced by Ang II (). These results showed that incubation of cultured podocytes with Ang II decreased the mRNA levels of CD2AP and that addition of Losartan prevented podocytes from decreasing CD2AP levels in response to Ang II. Our results showed that Losartan significantly increased the CD2AP mRNA levels (P < 0.05 versus Ang II). This suggests that Losartan may inhibit podocyte apoptosis induced by Ang II via CD2AP.
Table 1. CD2AP mRNA levels at different time.
Western blot analysis
CD2AP plays a central role in Ang II-induced apoptosis, To elucidate the mechanisms of Ang II-induced apoptosis in podocytes and the potential effects of Losartan on specific apoptotic ways, we performed Western blot analysis to measure the protein expression of CD2AP. Western blot analysis showed that in the control, Ang II and Losartan group, CD2AP protein expression had no changes at 8h (p > 0.05), incubation of the podocytes with Ang II markedly decreased CD2AP protein levels at 24 and 48h. In contrast, Losartan prevented the decrease in CD2AP induced by Ang II ( and ). These results demonstrate that incubation of cultured podocytes with Ang II decreased the protein levels of CD2AP and that addition of Losartan prevented podocytes from decreasing CD2AP levels in response to Ang II. Our results showed that Losartan significantly increased the CD2AP protein expression (P < 0.05 versus Ang II). This suggests that Losartan may inhibit podocyte apoptosis induced by Ang II via CD2AP protein expression.
Figure 1. Western blot band of CD2AP of control group, Ang II group and Losartan group at different time points. (a): The control, (b): AngII, (c): Losartan.
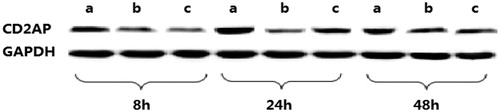
Table 2. CD2AP protein levels at different time.
Apoptosis analysis
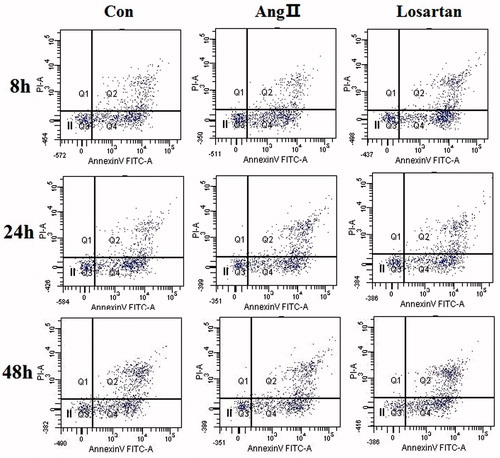
We first tested the hypothesis that Losartan has an inhibitory effect on Ang II-induced apoptosis in podocytes. To test this hypothesis, we quantified podocyte apoptosis and measured viable cell number in cultured immortalized podocytes that were grown under restrictive conditions for 14d and then were injured by exposure to Ang II. We first measured the percentage of apoptotic cells. Our results showed that Losartan significantly decreased the number of apoptotic podocytes induced by Ang II (P < 0.05 versus control). Our results confirmed that there was an increase in apoptotic cell number after 48h of exposure to Ang II. In contrast, Losartan significantly reduced Ang II-induced apoptosis at 48 h (). These results show that Ang II-induced podocyte apoptosis is Ang II dependent and supports the notion that Losartan exerts an antiapoptotic effect on cells that are exposed to Ang II through the regulation of Losartan.
Figure 2. The percentage of apoptosis measured by Annexin-V FITC and PI staining. PI and Annexin V apoptosis kit by double staining was used to detect the apoptosis rate and subjected to flow cytometric analysis, in which X axis is Annexin V(+) and Y axis is PI (+), Q1 is PI (+), Annexin (−V); Q2 is PI (+), Annexin (+V), the late apoptosis or dead cells; Q3 is PI (−), Annexin V (−), the normal living cell; Q4 is Annexin V (+), PI (−), the early apoptosis cells. Losartan prevents Ang II-induced apoptosis. The percentage of apoptotic cells, measured by Annexin-V FITC and PI staining increased at 24 h in the cells that were exposed to Ang II without Losartan, whereas cells that were treated with Losartan were resistant to Ang II-induced apoptosis. *p < 0.05 versus Losartan (–) Ang II (+).
Discussion
Podocytes are highly differentiated cells, once damaged and hard to be repaired, so the pathological changes of many kidney diseases showed as foot processes fusion and loss, podocytes injury and exfoliation. Accordingly, protecting podocytes is an important way to prevent kidney diseases or block the related progression. Clinically, the acquired podocyte diseases such as idiopathic familial focal segmental glomerulosclerosis (FSGS) and minimal change nephrotic syndrome (MCNS) were thought to be immune diseases, so commonly treated with immunosuppressive agents such as corticosteroids (GC) and calcineurin inhibitor (CNI). Future research should focus on defining the molecular targets, and the specific strategies for therapeutic intervention in podocyte disease. Special consideration should be given to optimizing modes of local delivery of therapies.Citation1–3 Podocyte injury is closely associated with the development of glomerulosclerosis in glomerular disease. Podocyte depletion as a result of apoptosis and the inability of podocytes to replicate cause glomerulosclerosis.Citation4 Seminal studies showed that podocyte apoptosis is a major factor causing a decrease in podocyte number.Citation5–8
CD2AP, the key proteins of podocyte slit diaphragm (SD), together form a signal transduction complex by interacting with other SD molecules and also play an important role in maintaining SD structural and functional integrity.Citation9–11 Data suggest that podocyte injury or SD structural damage will inevitably result in proteinuria. Proteinuria is a hallmark of loss of the glomerulus' permselectivity.Citation12 The growing number of studies showed that the SD is involved in the occurrence and transmission of signal transduction which maintained podocyte normal biological function, such as proliferation, differentiation, survival, endocytosis and cytoskeletal composition.Citation13–15 Therefore, SD which acts as a static molecular sieve and the highly dynamic complex, had a key effect on maintaining the normal structure of podocyte and functional integrity of the glomerular filtration barrier.Citation16 A recent report showed that glomerular mRNA level of CD2AP was significantly decreased in children with minimal change nephritic syndrome and primary IgA nephropathy. And a novel homozygous CD2AP mutation happened in a patient with FSGS. These findings addressed the important role of CD2AP in maintaining podocyte function.Citation17,Citation18
In this study, our data show that CD2AP played an important role in structural proteins and signaling proteins. And further illustrated the signal transduction mechanism of podocyte apoptosis induced by Ang II, Losartan prevents podocyte apoptosis induced by Ang II. Losartan exerts its antiapoptotic effect at the level of CD2AP. RT-PCR found that CD2AP mRNA levels significantly decreased after injury induced by Ang II, Western blot showed that there is no significance different in CD2AP protein levels between Ang II and the control group at 8h (P > 0.05), but it obviously decreased at 24 h and 48 h in Angα group (P < 0.05). The percentage of apoptotic cells, measured by Annexin-δ FITC and PI staining, increased at 24 h in the cells that were exposed to Ang II without Losartan, whereas cells that were treated with Losartan were resistant to Ang II-induced apoptosis, which may related to the decreased of CD2AP mRNA and protein expressions, Losartan may reduced the apoptotic podocytes by increased CD2AP mRNA and protein expressions. Losartan significantly protected podocytes from apoptosis induced by Ang II. This is very interesting; our study demonstrated the preventive effect of Losartan on podocyte apoptosis. However, further studies are required to delineate other molecular mechanisms of podocyte apoptosis and how Losartan has a direct protective effect on podocytes. A detailed understanding of the signaling events that occur at the cell-cell contacts of podocytes will help us to understand the pathogenesis of proteinuria. These findings provide a novel rationale for the use Losartan in clinical practice characterized by podocyte injury.
In summary, the major goal of this study was to explore the role of CD2AP in Ang II-induced podocyte injury and Proteinuria occurrence. Our present study revealed a novel molecular mechanism of Ang II-induced podocyte injury and proteinuria, which was associated with CD2AP down-regulation. We found that Ang II caused CD2AP functional changes in podocytes, such as lowered expression of CD2AP, and increased apoptosis. These results demonstrated that the direct harmful effect of Ang II on podocytes was mainly mediated by a mechanism that involved downregulation of CD2AP. These novel findings provide new insights into the podocyte injury and Proteinuria occurrence.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Lorz C, Benito-Martin A, Justo P, et al. Modulation of renal tubular cell survival: Where is the evidence? Curr Med Chem. 2006;13:763–771
- Qi R, Li W, Yu S. FK506 inhibits the mice glomerular mesangial cells proliferation by affecting the transforming growth factor-β and Smads signal pathways. Ren Fail. 2014;36:589–592
- Shankland SJ. The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147
- Yu SY, Qi R, Zhao H. Losartan reverses glomerular podocytes injury induced by AngII via stabilizing the expression of GLUT1. Mol Biol Rep. 2013;40:6295–6301
- Green DR, Kroemer G. Pharmacological manipulation of cell death: Clinical applications in sight? J Clin Invest. 2005;115:2610–2617
- Riedl SJ, Salvesen GS. The apoptosome: Signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413
- Fischer U, Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005;12:942–961
- Ortiz A, Gonzalez-Cuadrado S, Lorz C, Garcia del Moral R, O'Valle F, Egido J. Cytokines and Fas regulate apoptosis in murine renal interstitial fibroblasts. J Am Soc Nephrol. 1997;8:1845–1854
- Ding G, Reddy K, Kapasi AA, et al. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol. 2002;283:F173–F180
- Sanwal V, Pandya M, Bhaskaran M, et al. Puromycin aminonucleoside induces glomerular epithelial cell apoptosis. Exp Mol Pathol. 2001;70:54–64
- Suzuki T, Takemura H, Noiri E, et al. Puromycin aminonucleoside induces apoptosis and increases HNE in cultured glomerular epithelial cells. Free Radic Biol Med. 2001;31:615–623
- Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39
- Dobrinskikh E, Okamura K, Kopp JB, et al. Human podocytes perform polarized, caveolae-dependent albumin endocytosis. Am J Physiol-Renal Physiol. 2014;306:F941–F951
- Sonneveld R, van der Vlag J, Baltissen M, et al. Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am J Pathol. 2014;184:1715–1726
- Yu SY, Qi R. Role of bad in podocyte apoptosis induced by puromycin aminonucleoside. Transplant Proc. 2013;45:569–573
- Xing CY, Saleem MA, Ni L, Mathieson PW. Dexamethasone increases the proliferation and differentiation of human cultured podocytes [Abstract]. J Am Soc Nephrol. 2000;11:468A–322A
- Tory K, Menyhárd DK, Woerner S, et al. Mutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndrome. Nat Genet. 2014;46:299–304
- Ransom RF, Lam NG, Hallett MA. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 2005;68:2473–2483

