Abstract
Aim: Treg cells are of critical importance for maintenance of tolerance. The purpose of the this study was to observe the number of
Treg cells in the patients with thrombotic thrombocytopenic purpura (TTP) associated with systemic lupus erythematosus (SLE), and to study pathogenesis of TTP with SLE. Methods: Seven patients with TTP associated with SLE and seven healthy volunteers were studied. The
Treg cells were examined by flow cytometry. Clinical and laboratory data, such as urinary protein, serum creatinine, endothelial markers and immunologic serologics, were obtained from each patient and healthy volunteer. Glomerular injury was assessed by histopathology. Serum IL-2, IL-4, IL-6 and anti-endothelial cell antibody were analyzed by ELISA and anti-ADAMTS13 antibody were detected by Western blotting. Results:
Treg cells significantly decreased in TTP with SLE patients compared with controls (p < 0.05).
Treg cells are negatively correlated with blood urea nitrogen, serum uric acid, supernatant IL-4, and proteinuria, and positively with estimated glomerular filtration rate (eGFR) in TTP with SLE patients.
Treg cells gradually decreased as the severity of renal histology increased. Serum IL-2, IL-6, supernatant IL-4, anti-endothelial cell antibody, and anti-ADAMTS13 antibody significantly increased in TTP with SLE patients compared to those of the control groups (all p < 0.05). In contrast, serum levels of C3 were significantly decreased in TTP with SLE patients compared to those of the control groups (p < 0.05). Conclusions:
Treg cells are not only lower in TTP with SLE patients, but also are correlated with disease severity in TTP with SLE patients.
Treg cells may play an important role in the pathogenesis of TTP with SLE.
Introduction
TTP is an uncommon thrombotic microangiopathy (TMA) characterized with a clinical pentad: thrombocytopenia, microangiopathic hemolytic anemia (MAHA), fever, neurological deficits and renal dysfunction. Apart from some idiopathic cases, TTP may occur secondary to infections, malignancy, drugs, pregnancy and autoimmune diseases such as SLE.Citation1 Although plasma exchange dramatically improved the prognosis of TTP with over 80% of survival rate,Citation2 the episode will be severe and lethal (from 34.1 to 62.5% mortality rate) when TTP occurs in patients with SLE(referred as sTTP).Citation3,Citation4 In the last two decades, autoimmunity or vasculopathy with endothelial damage and platelet aggregation was considered as the pathogenesis of TTP in the setting of SLE.Citation4,Citation5 In recent years, an autoimmunity mechanism was proposed to be more important to the onset of TTP in the context of SLE, which was supported by the presence of various antibodies such as anti-endothelial cell antibody, antiplatelets antibody,Citation3 and anti-ADAMTS13 (von Willebrand factor cleaving metalloprotease) antibody.Citation6 However, the presence of various antibodies is still unclear until now.
Recent studies have shown that Treg cells are of critical importance to the maintenance of tolerance by inhibiting the activation and proliferation of autoreactive T cells.Citation7 Depletion of the minor (about 10%)
Treg cells results in the development of organ-specific autoimmunity.Citation8 Autoimmune diseases can be prevented by reconstitution of the animals with
Treg cells.Citation8 Powrie and colleaguesCitation9 demonstrated that transfer of
Treg cells protected mice from the development of inflammatory bowel disease and even reversed established gastrointestinal inflammation.
Treg cells are regulators in almost all of the animal models of human organ specific diseases, transplant rejection and allergic diseases.Citation10
To date, there are only few studies of Treg cells in human TTP associated with SLE. Some patients do not suffer from TTP associated with SLE, implying that it is possible to find a balance between immunity and tolerance. However, other patients do suffer from TTP associated with SLE. We, therefore, hypothesize that a numerical and/or functional deficit of
Treg cells in the TTP associated with SLE patients might trigger the development of disease. The purpose of the present study was to observe the number of
Treg cells in the patients with TTP associated with SLE.
Methods
Subjects
TTP associated with SLE patient groups
Seven patients with biopsy-proven lupus nephritis (LN), hospitalized in Hunan Provincial People’s Hospital and Second Xiangya Hospital of Central South University Hospital between January 2004 and February 2013 and who had typical features of TTP (1 men and 6 women; age range 25–47 years; mean age 37.8 ± 9.68 years), were enrolled. All patients satisfied the 1997 American College of Rheumatology criteria for SLE. They displayed microangiopathic hemolytic anemia, thrombocytopenia, neurologic syndrome, and renal impairment. Febrile patients with infection (e.g., pulmonary infection, biliary tract infection, or urinary tract infection) were excluded. Other factors that could lead to thrombotic microangiopathy (TMA), including malignant hypertension,Citation11–13 cyclosporine A toxicity, liver cirrhosis, cancer, and postpartum renal failure, were also excluded.
Control groups
Seven healthy volunteers (1 men and 6 women; age range 32–49 years; mean age 40.5 ± 6.75 years) were selected as the control group in this study.
The study was approved by the Ethical Committee of Hunan Provincial People’s Hospital and Second Xiangya Hospital of Central South University Hospital. Written informed consent was provided by all the patients and healthy volunteers.
Clinical data and clinical samples collected
After acquiring informed consent, clinical data, peripheral blood and urine samples were collected. All patients did not undergo treatment with steroid, immunosuppressive agents, angiotensin-converting enzyme (ACE) inhibitor, or AT1 receptor blockers before clinical samples collected. The laboratory examinations before treatments included urinalysis, complete blood count, serum chemistries, endothelial markers [von Wille brand factor (vWF), vascular cell adhesion molecule (VCAM)], and immunologic serologics (anti nuclear antibody, anti-double-stranded DNA antibody, anti-neutrophil cytoplasmic antibody, anti-endothelial cell antibody, and complement components C3).
Flow cytometry analysis of  Treg cells
Treg cells
Peripheral blood were mixed and incubated for 30 min at room temperature with 10 μL monoclonal Cy5-labeled anti-human CD3 (Jingmei Biotech), FITC-anti-CD4, and PE-anti-CD25. After a short incubation period, the samples were fixed with 1% paraformaldehyde and analyzed by flow cytometry (Coulter EpicsXL, System2 software; Beckman-Coulter). The analysis and gates were restricted to lymphocytes (the number of replicates of each sample was three).
Isolation of peripheral blood mononuclear cells and culture
PBMC were isolated from heparinized peripheral blood by density gradient centrifugation, using Lymphocyte Separation Medium (Flow Labs, McLean, VA). Cells recovered at the interface were resuspended in RPMI1640 supplemented with penicillin (100 U/mL), streptomycin (100 pg/mL), glutamine (2 mM) and 10% heat-inactivated fetal calf serum (FCS), at a concentration of 3 × 106 cells/mL. Duplicate cultures, with phytohemagglutinin (PHA; Sigma, St Louis, MO) 20 ug/mL, were maintained for 24 h at 37 °C in a 5% CO2 atmosphere. At the end of this period, cell-free supernatant was obtained by centrifugation at 800 g for 10 min and frozen at −70 °C until assayed.
Enzyme-linked immunosorbent assay
The serum concentrations of IL-2, IL-6, anti-endothelial cell antibody, and the cell-free supernatant concentration of IL-4 were measured respectively by an enzyme-linked immunosorbent assay (ELISA) with ELISA kits (R&D Systems, Cambridge, MA) according to the manufacturer’s instruction (the number of replicates of each sample was three).
Western blotting for anti-ADAMTS13 antibody
Anti-ADAMTS13 antibodies were searched for by Western blot analysis. The supernatant of cells producing recombinant ADAMTS13 as previously describedCitation14 (100 ng/Lane) was added to gel-loading buffer (50 mM Tris-HCl pH 6.8, 2% sodium dodecyl sulphate, 0.1% bromophenol blue, 10% glycerol). Next, 7% sodium dodecyl sulphate polyacryl gel electrophoresis was performed in Tris-glycine buffer, pH8.3 and migrated samples were transferred onto a pure nitrocellulose membrane (Bio-Rad, Hercules, CA). After blocking with skimmed milk, membranes were incubated with citrated plasma samples (diluted 1/100 in TBS, 5% skimmed milk, 0.05% Tween 20, pH 7.4) used as a possible source of anti-ADAMTS13 antibody. Bound anti-bodies were visualized using alkaline phosphatase-labeled anti-human immunoglobulin (diluted 1/2000) and an alkaline phosphatase conjugate substrate kit (Amersham Bioscences, Uppsala, Sweden). β-Actin was probed as a control for equal protein loading (the number of replicates of each sample was three).
Renal histopathology examination
Renal biopsy was performed in 7 TTP with SLE patients. All kidney biopsy samples were subjected to fluorescence, and light microscopic examination. For light microscopy, kidney biopsy samples were fixed in 10% buffered formalin, dehydrated, and embedded in paraffin by conventional techniques. Sections were stained with hematoxylin and eosin, and periodic acid-schiff. IgG, IgA, IgM, complement component C3, activity (AI) and chronicity (CI) indices, as well as semiquantitative renal histological evaluation of renal biopsy specimens obtained at time of diagnosis, were compared. AI includes glomerular hypercellularity, leucocyte exudation, fibrinoid necrosis, cellular crescents, hyaline deposits and interstitial inflammation, while CI includes glomerular sclerosis, fibrous crescents, renal tubular atrophy and interstitial fibrosis. These alterations were graded semiquantitatively on a 1+ to 3+ scale (mild, moderate, or marked).Citation15 The renal pathological lesions were graded single-blinded according to the Haas classification.Citation16
Statistical analysis
Data were checked for normality of distribution using the Kolmogorov–Smirnov test and were expressed as mean standard deviation or median and interquartile range. Comparison between the two groups was done by independent-sample t-test or non-parametric Mann–Whitney U-test as appropriate. Relationships between different values were examined using Spearman’s correlation tests. A p value <0.05 was considered statistically significant. All statistical analyses were performed using Graph Pad Prism 5.0 (Graph Pad Inc., Boston, MA) and Statistical Package for Social Sciences version 16.0 (SPSS Inc., Chicago, IL).
Results
Flow cytometry analysis of  Treg cells
Treg cells
We analyzed the population of Treg cells in peripheral blood by flow cytometry ().
Treg cells significantly decreased in TTP with SLE patients compared to those of the control groups (ap < 0.05) ().
Figure 1. Treg cells in peripheral blood by flow cytometry. Notes:
Treg cells were counted using the indicated gates and are enumerated in . α:
Treg cells, I: TTP with SLE patients, II: Control groups.
Treg cells significantly decreased in TTP with SLE patients compared to the control Groups (p < 0.05).
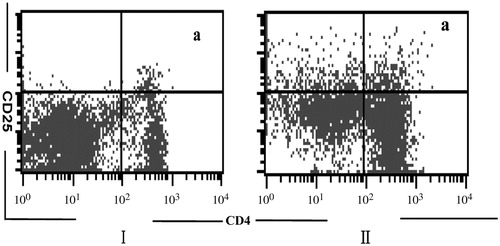
Table 1. The number of Treg cells in peripheral blood (mean ± SD)%.
Clinical and histopathological findings in patients
Clinical and histopathological findings in patients were listed in . Serum IL-2, IL-6, supernatant IL-4, anti-endothelial cell antibody, anti-ADAMTS13 antibody, VCAM and vWF significantly increased in TTP with SLE patients compared to those of the control groups (all *p < 0.05). In contrast, serum levels of C3 were significantly decreased in TTP with SLE patients compared to those of the control groups (*p < 0.05).
Table 2. Clinical and histopathological findings in TTP with SLE patients.
 Treg cells and clinical data
Treg cells and clinical data
The correlations between Treg cells and values for numerous clinical parameters were examined in TTP with SLE patients ().
Treg cells were negatively correlated with serum blood urea nitrogen (BUN), and uric acid in TTP with SLE patients, and
Treg cells were positively correlated with eGFR (all *p < 0.05). These results indicate that
Treg cells were associated with renal function.
Treg cells were negatively correlated with urine protein, supernatant IL-4 in TTP with SLE patients (all *p < 0.05).
Table 3. Correlation between the frequency of Treg cells and clinical parameters in TTP with SLE patients.
Correlation between  Treg cells and histological classification of TTP with SLE
Treg cells and histological classification of TTP with SLE
Treg cells in TTP with SLE patients tended to decrease in parallel with the severity of the histological classification of nephropathy as determined by the Haas classification,Citation16 although the difference was not significant (all p > 0.05).
Discussion
The pathogenesis of SLE complicated with TTP is still unclear, although some factors such as heredity, infection, drug, hyper sensitivity, unique immunology, tumor, pregnancy, and hematopoietic stem cell transplant might be responsible.Citation17–19 Treg cells are regulators in almost all of the animal models of human organ specific diseases, transplant rejection and allergic diseases.Citation10 The most notable immunomodulatory property of
Treg cells is their ability to limit the development of a proinflammatory CD4+ Th2 phenotype;Citation20 this inhibition is characterized by reduced cytokine production. Abnormality of peripheral T cell can result from an inappropriate balance between allergen activation of
Treg cells and effector Th2 cells.Citation21,Citation22 This imbalance could result from a deficiency in suppression by
Treg cells or strong activation signals that overcome such regulation.Citation23 Recent work has shown that following antigen inhalation,
Treg cells play a key immunomodulatory role.Citation24 It has been reported that Th1 responses are more prone to regulation by
Tcells than Th2 responses.Citation25 Some patients do not suffer from TTP with SLE, implying that it is possible to find a balance between immunity and tolerance. However, other patients do suffer from TTP with SLE. We therefore hypothesize that a numerical and/or functional deficit of
Treg cells in the TTP with SLE patients might trigger the development of disease. The purpose of the present study was to observe the number of
Treg cells in the TTP with SLE patients.
Treg cells significantly decreased in TTP with SLE patients compared with controls. Our results are consistent with this suggestion. Serum IL-2, IL-6, and supernatant IL-4, anti-endothelial cell antibody, anti-ADAMTS13 antibody, VCAM and vWF, urine protein, and urine erythrocytes significantly elevated in TTP with SLE patients compared with controls.
Treg cells were negatively correlated with serum BUN, uric acid, supernatant IL-4 and urinary protein, and were positively correlated with eGFR. The
Treg cells tended to gradually decrease with increasing severity of histologically assessed nephropathy. When TTP with SLE patients experiences a decrease in
Treg cells, his/her lymphocytes react significantly more strongly to antigens, leading to higher levels of cytokine production (IL-2, IL-4, IL-6). Excessive cytokine production may enhance the production of antibody such as anti-endothelial cell antibodies, anti-ADAMTS13 antibodies. Therefore, higher levels of cytokine and large amounts of antibody are present in the blood circulation where they can directly injury vascular endothelial (elevated levels of endothelial functional parameters, such as von Wille-brand factor, VCAM), make platelet abnormalities, and activate complement or disturb the balance between blood coagulation and plasminogen, and may contribute to the development of microvascular thrombosis that causes variable signs and symptoms of organ ischemia and damage.Citation26–30
Treg cells may play an important role in the pathogenesis of TTP with SLE. To date, there are only few studies of
Treg cells in human TTP with SLE.
Although some findings mentioned above are both interesting and useful, limitation is obvious. The relatively fewer cases limited the statistical power for comparisons.
In conclusion, the present study indicated that Treg cells are not only lower in TTP with SLE patients, but also are correlated with disease severity in TTP with SLE patients.
Treg cells may play an important role in the pathogenesis of TTP with SLE. Additional studies are necessary to demonstrate a functional deficit of
Treg cells in TTP with SLE patients and other biological properties of
Treg cells.
Declaration of interest
This study was supported in part by the foundation of Beijing Shijitan Hospital, Capital Medical University (No. 2011-C06), Hunan Provincial Natural Sciential Foundation (No. 09jj6056), and the Foundation for the Excellent Doctoral Education of Central South University (No. 2340) China.
All the authors have declared no competing interest.
References
- Veyradier A, Meyer D. Thrombotic thrombocytopenic purpura and its diagnosis. J Thromb Hemost. 2005;3:2420–2427
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hemat Am Soc Hematol Educ Program. 2004;3:407–423. doi:10.1182/asheducation-2004.1.407
- Letchumanan P, Ng HJ, Lee LH, Thumboo J. A comparison of thrombotic thrombocytopenic purpura in an inception cohort of patients with and without systemic lupus erythematosus. Rheumatology (Oxford). 2009;48:399–403
- Musio F, Bohen EM, Yuan CM, Welch PG. Review of thrombotic thrombocytopenic purpura in the setting of systemic lupus erythematosus. Semin Arthritis Rheum. 1998;28:1–19
- Zheng T, Chunlei L, Zhen W, et al. Clinical-pathological features and prognosis of thrombotic thrombocytopenic purpura in patients with lupus nephritis. Am J Med Sci. 2009;338:343–347
- Lansigan F, Isufi I, Tagoe CE. Microangiopathic hemolytic anaemia resembling thrombotic thrombocytopenic purpura in systemic lupus erythematosus: The role of ADAMTS13. Rheumatology (Oxford). 2011;50:824–829
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562
- Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by suppressor T cells. Immunol Rev. 2001;182:58–67
- Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–98 (discussion 2003;98–105:106–114)
- Shi H-Z, Qin X-J. D4+CD25+ regulatory T lymphocytes in allergy and asthma. Allergy. 2005;60:986–995
- Desch KC, Motto DG. Thrombotic thrombocytopenic purpura in humans and mice. Arterioscler Thromb Vasc Biol. 2007;27:1901–1908
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725
- Matsumoto M, Yagi H, Ishizashi H, Wada H, Fujimura Y. The Japanese experience with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Semin Hematol. 2004;41:68–74
- De Cristofaro R, Peyvandi F, Palla R, et al. Role of chloride ions in modulation of the interaction between von Willebr and factor and ADAMTS-13. J Biol Chem. 2005;280:23295–23302
- Jiang L, Liu G, Lv J, et al. Concise semiquantitative histological scoring system for immunoglobulin A nephropathy. Nephrology (Carlton). 2009;14:597–605
- Haas M. Histologic subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–842
- Franchini M, Zaffanello M, Veneri D. Advances in the pathogenesis, diagnosis and treatment of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Thromb Res. 2006;118:177–184
- Lowe E, Werner E. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome in children and adolescents. Semin Thromb Hemost. 2005;31:717–730
- Zakarija A, Bennett C. Drug-induced thrombotic microangiopathy. Semin Thromb Hemost. 2005;31:681–690
- Mottet C, Uhlig HH, Powrie F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004
- Thornton AM, Shevach EM. Suppressor effector function of immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190
- Huang H, Peng Y, Liu F, Lei H. Is IgA nephropathy induced by abnormalities of Treg cells in the tonsils? Med Hypotheses. 2007;69:410–413
- Akbari O, Freeman GJ, Meyer EH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032
- McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: A novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–231
- Boumpas DT, Fessler BJ, Austin HA, Balow JE, Klippel JH, Lockshin MD. Systemic lupus erythematosus: Emerging concepts. Ann Intern Med. 1995;123:42–53
- D’Cruz DP, Houssiau FA, Ramirez G, et al. Antibodies to endothelial cells is sytemic lupus erythematosus: A potential marker for nephritis and vasculitis. Clin Exp Immunol. 1991;85:254–261
- Perry GJ, Elston T, Khouri NA, Chan TM, Cameron JS, Frampton G. Antiendothelial cell antibodies in lupus: Correlations with renal injury and circulating markers of endothelial damage. Q J Med. 1993;86:727–734
- Chu R, Russell NH, Powell RJ, Cater DR, Harris RJ. Abnormal fibrinolytic activity in systemic lupus erythematosus and possible mechanisms. Br J Rheumatol. 1988;27:436–439
- Awada H, Barlowatz-Meimon G, Dougados M, Maisonneuve P, Sultan Y, Amor B. Fibrinolysis abnormalities in systemic lupus erythematosus and their relation to vasculitis. J Lab Clin Med. 1988;111(2):229–236

