Abstract
Background: We aimed to evaluate whether serum apelin could reflect the nutritional status of children on dialysis. Methods: Twelve patients on peritoneal dialysis (PD) and 20 patients on hemodialysis (HD) were enrolled. Patients received individualized diet for six months. Anthropometric and laboratory indices were measured at onset and the end of the study. Results: The anthropometric indices were all significantly lower in patients than in controls whereas similar in PD and HD patients. The protein catabolic rate (nPCR), height, mid-arm circumference (MAC), triceps skinfold thickness (TSF), arm muscle area (AMA) and arm fat area (AFA) z scores were significantly increased in dialysis patients after nutritional intervention. Weight z scores statistically increased in HD group whereas did not statistically change in PD group. Serum albumin levels were significantly improved in PD and HD patients. Apelin levels were similar in PD, HD and control groups. Post nutritional apelin values did not differ in each dialysis groups. On multivariate analysis, apelin was independently associated with age, weight, ESR and TG. Conclusions: Apelin seems to be not a useful indicator for monitoring the nutritional status in children on dialysis. However, the close link of apelin with inflammatory and lipid parameters suggested that apelin might be a novel target for slowing the atherogenic process in pediatric dialysis patients.
Introduction
Malnutrition represents an important source of morbidity and mortality in chronic kidney disease (CKD). Nearly 20% to 75% of adult dialysis patients suffer from protein-energy wasting or cachexia.Citation1,Citation2 Also, malnutrition is highly prevalent in children with CKD and tends to increase when dialysis is initiated. Anorexia, dietary restrictions, increased catabolism, micro-inflammation, oxidative stress, loss of nutrients, insulin resistance and altered insulin signaling all contribute to uremic malnutrition.Citation3–5
Over the last decade, adipose tissue was introduced to be a new endocrine organ. Not withstanding fat-storage, it also secretes adipokines, cytokines, hormones and hormone like peptides participating in the regulation of food intake, energy expenditure, metabolism, inflammation, endothelial health and blood pressure homeostasis.Citation6,Citation7 In uremic milieu, due to reduced renal clearance of adipokines, adipose tissue becomes a significant contributor to increased systemic inflammation and uremic anorexia.Citation8,Citation9
Apelin is a novel adipocytokine, also expressed in kidney, heart, central nervous system and endothelium. It was identified as the endogenous ligand of an orphan G protein-coupled receptor (APJ) which bears closest identity to the angiotensin II type 1 receptor (AT-1). Apelin/APJ system plays role in the regulation of cardiovascular, gastrointestinal, immune functions, as well as on fluid homeostasis, angiogenesis and bone physiology.Citation10,Citation11 Apelin is reduced in patients with heart failure and up regulated following left ventricular remodelling. The inotropic, vasodilatator features as well as homeostatic effects of apelin in fluid balance make it a favorable target for heart failure therapy.Citation12 Apelin increases in obesity and positively correlates with body mass index (BMI). Furthermore apelin stimulates glucose utilization, decreases insulin secretion and negatively regulates catecholamine-mediated lipolysis.Citation13,Citation14 Recently clinical and experimental studies indicated a link between apelin and inflammation.Citation15–18 Plasma apelin has been shown to negatively correlate with inflammatory markers in hemodialyzed patients.Citation18
Based upon the evidence of malnutrition and inflammation and considering the putative role of adipose tissue in CKD, we aimed to evaluate whether serum apelin could reflect the nutritional status of children on peritoneal dialysis (PD) and hemodialysis (HD).
Material and methods
Patients and study design
This prospective study was conducted in pediatric nephrology clinic of Cukurova University. Age and gender matched 30 patients (12 PD, 18 HD) and 21 healthy children were included. Exclusion criteria were age over 18 years, presence of a prior major disease, systemic infections, peritonitis or mental retardation, history of surgical intervention or hospitalization for the last three months and use of immunosuppressive agents. The underlying renal pathology for CKD was dysplasia/hypoplasia with or without obstructive uropathy (n = 22), steroid resistant nephritic syndrome (n = 5), crescentic glomerulonephritis (n = 1), Bardet Biedl syndrome (n = 1) and oxalosis (n = 1). The project was approved by the ethics committee of Cukurova University Hospital and written informed consent was received from at least one of the parents in all cases.
At the onset of the study, an adequate dietary prescription meeting caloric and protein requirements was adjusted by an experienced dietician for each patient. The caloric intake was considered to be 100% of estimated energy requirement (EER) for chronological age and body size. When the patient’s usual dietary intake failed to meet his/her EER, supplemental nutritional support was added. A balance of calories from macronutrients was distributed as carbohydrate 45–65%, protein 10–30% and fat 25–35%. The dietary protein intake was calculated to be 100% of daily recommended intake (DRI) for ideal body weight plus an allowance for dialytic protein and amino acid losses (0.1 g/kg/d for HD, 0.15–0.3 g/kg/d for PD). The protein supplements were prescribed when the oral foods and fluids were unable to meet protein requirements. Total calcium (Ca) intake from nutrients or phosphate (P) binders was determined in the range of 100–200% of the DRI for Ca for age. The dietary P was arranged according to serum P and target parathormone (PTH) level. Water soluble vitamins were routinely advised.Citation1
Patients were asked for keeping a dietary diary and taking on the individualized diet for the next six months. The same dietician weekly interviewed with the children and controlled the diaries. Just before and at the end of 6 months of dietary intervention, anthropometric and laboratory tests were measured during the same study visit. Measurements were made 30 min after midweek dialysis session in HD patients and after drainage of peritoneal fluid in PD patients.
Anthropometry
Anthropometric measurements were made in euvolemic state by the same experienced dietitian using standard techniques according to the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines.Citation1 The standard deviation score (SDS) of weight/age, height/age and body mass index (BMI) were analyzed by using the least-mean-squire (LMS) method from the references for Turkish children.Citation19,Citation20 Malnutrition was defined as having weight for height less than −2 z scores.
Mid-arm circumference (MAC) was measured in the non-fistula upper arm midway between the acromion and the olecranon process with a flexible measuring type. Triceps skinfold thickness (TSF) was measured at the posterior midpoint of the right upper arm using a skinfold caliper. Two measurements for TSF were obtained and the average was recorded. The arm fat area (AFA) and arm muscle area (AMA) was calculated by means of the standard equation.Citation21 SDS values of TSF, MAC and AFA for age and height age were determined according to the National Health and Nutrition Examination Survey (NHANES) percentile distribution tables.Citation22
The blood pressure (BP) of the participants was measured three times consecutively by a mercury sphygmomanometer following 5 minutes rest time and average of the values was taken. To standardize BP for differences in age and height, z scores were calculated according to data given in Forth Report of Task Force.Citation23
Protein catabolism
The protein catabolic rate (nPCR) is a quantitative measure of protein catabolism and calculated by urea kinetic modeling in HD and by multiplying the sum of urinary and dialysate urea nitrogen using the formula in PD patients.Citation24,Citation25
Assays
Blood samples were obtained for determination of apelin level, serum biochemistry and hemogram. Samples were analyzed by using routine laboratory methods for hemoglobin (Hb), erythrocyte sedimentation rate (ESR), blood urea nitrogen (BUN), serum creatinine (Scr), total protein, albumin, prealbumin, Ca, P, total cholesterol, HDL, LDL and VLDL cholesterol, triglyceride (TG), C-reactive protein (CRP) and PTH.
The samples for apelin analysis were frozen in 2-ml tubes and stored at −80 °C until the test day. Serum apelin concentrations were measured with Apelin-36 (Human)-EIA kit (Phoenix Pharmaceuticals, Inc, Burlingame, CA) and a solid-phase, two-side chemiluminescent enzyme immunometric assay (Immulite 1000 automated analyzer; Siemens Medical Solutions Diagnostics, Los Angeles, CA).
Statistics
Statistical analysis was done by using SPSS version 18.0 (Chicago, IL) for Windows. Assumptions of normality and homogeneity of variance were initially checked. Data were expressed as median with interquartile range (IQR). The differences between the groups were compared with using Mann–Whitney U or Kruskall–Wallis tests. The consecutive data of the same groups were analyzed by Wilcoxon test. Categorical variables were expressed as proportions and compared with using Chi-square test. Correlations between apelin and other variables were assessed by Spearman rank coefficient. A value of p ≤ 0.05 was considered statistically significant for all tests. Multiple regression analysis was used to determine independent variables affecting dependent factors. The variables showing a linear correlation with apelin and gender, PTH, SBP and DBP were tested in a multivariate analysis.
Results
The current study involved in 30 children on chronic dialysis and 21 age- and gender-matched healthy controls. Twelve patients aged 10.9 ± 6 years were on PD (8 on automated dialysis) and 18 patients aged 13.3 ± 3 years were on thrice-weekly HD. The mean time on dialysis was 32.2 ± 27 (6–79) months in PD and 35.3 ± 24.2 (6–84) months in HD patients (p = 0.74).
Two of the PD and 8 of the HD patients were completely anuric. The initial nPCR as well as SBP and DBP values were similar in dialysis groups (p = 0.76, p = 0.84 and p = 0.65, respectively).
shows demographic and anthropometric data of study groups. Malnutrition was present in 12 (40%) patients. All the anthropometric indices were significantly lower in patients than in controls (p = 0.001); however, there was no difference in the nutritional indices between PD and HD patients either at the beginning or the end of the study (p > 0.05; data not shown). The height, MAC, TSF, AMA and AFA z scores were significantly increased in dialysis patients after 6 months of nutritional intervention (p < 0.05). Weight z scores statistically increased in HD group (p = 0.047) whereas not significantly changed in PD group (p = 0.62). Post-nutritional BMI z scores did not significantly differ in the PD and HD patients (p = 0.56 and p = 0.286). The nPCR values were significantly improved in PD and HD groups following nutritional intervention (p = 0.002 and p = 0.001, respectively).
Table 1. Anthropometric data of study groups at baseline and after six months.
The initial and final laboratory variables of the patients and controls are displayed in . Patients had significantly lower hb and albumin, and significantly higher ESR, serum CRP, total cholesterol and TG levels compared to healthy controls (p = 0.001). Serum albumin levels were significantly lower in PD patients than in HD patients (p = 0.01). None of the other laboratory parameters differed in the PD and the HD patients (p > 0.05; data not shown). At the end of the study, serum albumin levels were statistically increased in both PD and HD patients (p = 0.037 and p = 0.05, respectively) whereas serum prealbumin levels were only significantly increased in PD patients (p = 0.034). Post nutritional lipid profile and serum apelin values did not statistically change in all dialysis groups (p > 0.05).
Table 2. Laboratory data of dialysis patients at baseline and after six months.
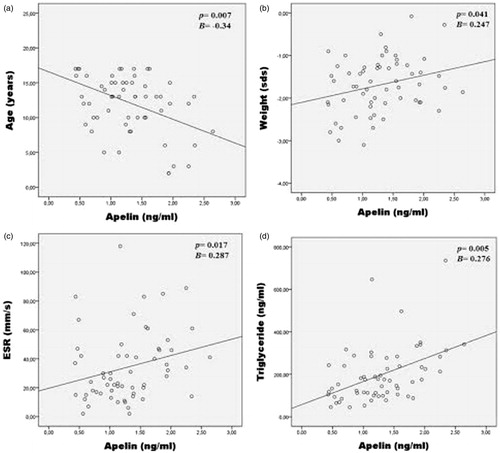
On univariate analysis, apelin was positively correlated with weight (p = 0.047; r = 0.360), ESR (p = 0.018; r = 0.304), CRP (p = 0.045; r = 0.442), total cholesterol (p = 0.005; r = 0.360), LDL (p = 0.002; r = 0.388) and TG (p = 0.001; r = 0.444); negatively correlated with age (p = 0.005; r = −0.359). Multivariate linear regression analysis showed that apelin was independently associated with age (Beta = −0.34, p = 0.007), weight (Beta = 0.247, p = 0.041), ESR (Beta = 0.287, p = 0.017) and TG (Beta = 0.276, p = 0.005; ).
Discussion
The present study re-indicated a high prevalence of malnutrition with an ongoing inflammatory and lipidogenic milieu in children on dialysis. Apelin seems to be not affected either by severe kidney dysfunction or nutritional intervention and might not be a useful indicator for monitoring the nutritional status in pediatric dialysis patients. However, the close link of apelin with inflammatory and lipid parameters suggested that apelin might be a novel target for slowing the atherogenic process in patients with CKD.
Apelin is an adipocyte derived cytokine which is involved in a variety of physiological functions.Citation10,Citation11 However, little is known about apelin in nephrology area, particularly in pediatrics. To our knowledge, this is the first study assessing apelin and its relation with nutritional indices in children with CKD. We found either presence of severe renal failure or mode of dialysis had no significant effect on serum apelin levels.
Literature data regarding plasma apelin concentrations in uremia is conflicting. Contrary to us, earliest studies reported lower apelin values in dialysis patients compared to healthy individuals and the decrease was more in those with more severe heart impairment.Citation18,Citation26 However, Codognotto et al. found lower apelin levels in HD patients with cardiomyopathy than in non-uremic precedents and concluded that uremia, not the severity of heart failure, was the determinant for apelin reduction.Citation27 On the other hand, in support to our results, three recent papers did not show a difference between apelin in dialysis patients and healthy individuals.Citation28–30 The discrepancy between previous and the latest reports might be due to various factors influencing apelin production/release and removal or the methodological heterogeneity of the studies. In accordance with Karadag et al.,Citation31 we also found age was inversely correlated with serum apelin levels. Thus, the negative impact of age on apelin might contribute to the identical results in our pediatric ESRD patients.
It has been shown that nutritional status regulates the expression of apelin in adipose tissue.Citation32 In the present study, although anthropometric and laboratory nutritional measurements significantly improved following dietary intervention, no significant change was observed in serum apelin levels. Moreover, apelin was not related with nutritional indices such as BMI, TAC, AMA, AFA, albumin and prealbumin. Likewise, Mafra et al. and Leal et al. did not find an association between apelin and adiposity markers.Citation28,Citation29 These evidences suggested apelin might not be a useful indicator of nutritional status in children on dialysis.
Recent reports described a link between apelin and volume status.Citation33,Citation34 However we did not directly analyze body fluid composition by bio-impedance spectroscopy (BIA). Although all measurements were done in euvolemic state, hypervolemia was not actually ruled out because of hypertension and hypoalbuminemia, particularly in PD patients. So, the independent association of apelin with weight was thought to be a reflection of hydration status.
The experimental studies suggested a putative role for apelin in inflammation.Citation15,Citation35 However, apelin-inflammation link is discordant in HD patients. In the current study, apelin was positively correlated with ESR and CRP in children on PD and HD. Malyszko et al. reported similar whereas El-Shehaby et al. and Cernaro et al. showed an inverse association between CRP and apelin in HD patients. On the other hand, Leal et al. and Codognotto et al. did not find any association between those parameters.Citation27,Citation28 These different results might be related with changes in the inflammatory profile of PD, HD, adult and pediatric populations.
The inhibitory effect of apelin on adipogenesis and lipolysis was previously demonstrated.Citation36,Citation37 In the present study, we detected positive correlation of apelin with total cholesterol, LDL cholesterol and TG as TG was one of the major determinants of apelin levels on multivariate analysis. Likewise, Karadag and Kazancioglu et al. reported positive association between apelin and LDL in PD patients.Citation31,Citation34 However, Malyszko et al. found apelin was negatively correlated with TG, total and LDL cholesterol in HD patients.Citation38 The controversy between those studies might be due to dialysis modality as PD patients are more predisposed to metabolic disturbances, particularly to hyperglycemia and dyslipidemia. On the other hand, novel papers focused on contribution of apelin to atherosclerosis triggered by dyslipidemia.Citation39–41 Despite the lack of cardiovascular and atherosclerotic investigations in our study, the strong relationship found between apelin and lipids suggested that apelin might be a target for preventing hyperlipidemia and subsequent atherosclerotic processes in patients with ESRD.
There are some limitations of this study. The sample size was relatively small and represented from a single center. The period of nutritional care might be short. Additionally, we could not analyze body composition with BIA because of its unavailability in our institution. The other limitation is lack of cardiac investigation. Comparison of apelin with atherosclerotic markers and echocardiographic indices would provide more information about its probable role on atherosclerosis.
In summary, apelin seemed to be not appropriate for assessing the nutritional status of children on dialysis. The strong relationship of apelin among inflammatory and lipid parameters suggested that apelin might be a novel target for slowing the atherogenic process in ESRD. Further studies are needed to shed more light on the probable role of apelin upon subsequent atherosclerosis in CKD.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The study was funded by the unit of Scientific Research Project in Cukurova University: TF 2009BAP36. The authors thank to Ruksan Anarat and her staff for their strenuous efforts on biochemical analysis, and also to Safak Oznergiz, Gulcan Delidag and Hanifi Saban for allocation and preservation of the serum samples.
References
- KDOQI Work Group. KDOQI Clinical Practice Guidelines for Nutrition in children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009;53(Suppl 2):S11–S104
- Mak RH, Cheung WW, Zhan JY, Shen Q, Foster BJ. Cachexia and protein-energy wasting in children with chronic kidney disease. Pediatr Nephrol. 2012;27:173–181
- Sylvestre LC, Fonseca KPD, Stinghen AEM, Pereira AM, Meneses RP, Pecoits-Filho R. The malnutrition and inflammation axis in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2007;22:864–873
- Kuhlmann MK, Levin NW. How common is malnutrition in ESRD? New approaches to diagnosis of malnutrition. Blood Purif. 2008;26:49–53
- Bonanni A, Mannucci I, Verzola D, Saffioti S, Gianetta E, Garibotto G. Protein-energy wasting and mortality in chronic kidney disease. Int J Environ Res Public Health. 2011;8:1631–1654
- Wosniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: The new endocrine organ? A review article. Dig Dis Sci. 2008;54:1847–1856
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919
- Axelsson J, Stenvinkel P. Role of fat mass and adipokines in chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:25–31
- Manolescu B, Stoian I, Atanasiu V, Busu C, Lupescu O. Review article: The role of adipose tissue in uremia-related insulin resistance. Nephrology. 2008;13:622–628
- Castan-Laurell I, Boucher J, Dray C, Daviaud D, Guigné C, Valet P. Apelin, a novel adipokine over-produced in obesity: Friend or foe? Mol cell Endocrinol. 2005;245:7–9
- Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211
- Vhandrasekaran B, Dar O, McDonagh T. The role of apelin incardiovascular function and heart failure. Eur J Heart Fail. 2010;10(8):725–732
- Laderidas-Lopes R, Ferreira-Martins J, Leite-Moreira AF. The apelinergic system: The role played in human physiology and pathology and potential therapeutic applications. Arq Bras Cardiol. 2008;90:343–349
- Xu S, Tsao PS, Yue P. Apelin and insulin resistance: Another arrow for the quiver? J Diabetes. 2011;3:225–231
- Daviaud D, Boucher J, Gesta S, et al. TNFalfa up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006;20:1528–1530
- Malyszko J, Malyszko JS, Pawlak K, Wolczynski S, Mysliwiec M. Apelin, a novel adipocytokine, in relation to endothelial function and inflammation in kidney allograft recipients. Transplant Proc. 2008;40:3466–3469
- Yu S, Zhang Y, Li MZ, et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin Med J (Engl). 2012;125:3440–3444
- El-Shehaby AM, El-Khatib MM, Battah AA, Roshdy AR. Apelin: A potential link between inflammation and cardiovascular disease in end stage renal disease patients. Scand J Clin Lab Invest. 2010;70:421–427
- Neyzi O, Andrzej F, Bundak R, Gunoz H, Draendeliler F, Bas F. Growth references for Turkish children aged 6–18 years. Acta Pediatr. 2006;95:1635–1641
- Bundak R, Furman A, Gunoz H, Darendeliler F, Bas F, Neyzi O. Body mass index references for Turkish children. Acta Pediatr. 2006;95:194–198
- Paglialonga F, Edefonti A. Nutrition assessment in children on peritoneal dialysis. Pediatr Nephrol. 2009;24:721–730
- Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1982;34:2540–2545
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576
- Srivaths PR, Wong C, Goldstein SL. Nutrition aspects in children receiving maintenance hemodialysis: Impact on outcome. Pediatr Nephrol. 2009;24:951–957
- Mendley SR, Majkowski NL. Urea and nitrogen excretion in pediatric peritoneal dialysis patients. Kidney Int. 2000;58:2564–2570
- Malyszko J, Malyszko JS, Koźminski P, Myśliwiec M. Apelin and cardiac function in hemodialyzed patients: Possible relations? Am J Nephrol. 2006;26:121–126
- Codognotto M, Piccoli A, Zaninotto M, Vertolli U, Tona F, Boffa GM. Evidence for decreased circulating apelin beyond heart involvement in uremic cardiomyopathy. Am J Nephrol. 2007;27:1–6
- Leal VO, Lobo JC, Stockler-Pinto MB, Farage NE, et al. Apelin: A peptide involved in cardiovascular risk in hemodialysis patients? Ren Fail. 2012;34:577–581
- Mafra D, Lobo JC, Farage NE, et al. The relationship between apelin and parathyroid hormone in hemodiaysis patients. Ren Fail. 2012;34:970–973
- Cernaro V, Lacquaniti A, Lorenzano G, et al. Apelin, plasmatic osmolality and hypotension in dialyzed patients. Blood Purif. 2012;33:317–323
- Karadag S, Ozturk S, Gursu M, et al. The relationship between apelin and cardiac parameters on peritoneal dialysis: Is there a new cardiac parameter? BMC Nephrol. 2014;15:18
- Boucher J, Masri B, Davidaud D, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771
- Charles CJ. Putative role for apelin in pressure/volume homeostasis and cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2007;5:1–10
- Kazancioglu R, Gursu M, Karadag S, et al. Volume status in patients on peritoneal dialysis: The role of apelin and bio-impedance spectroscopy. Ren Fail. 2012;34:1068–1073
- Garcia-Diaz D, Campion J, Milagro FI, Martinez JA. Adiposity dependent apelin gene expression: Relationships with oxidative and inflammation markers. Mol Cell Biochem. 2007;305:87–94
- Yue P, Jin H, Xu S, et al. Apelin decreases lipolyses via G(q), G(i), and AMPK-Dependent mechanisms. Endocrinology. 2011;152:59–68
- Than A, Cheng Y, Foh LC, et al. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol Cell Endocrinol. 2012;15:227–241
- Malyszko J, Kozminski P, Malyszko J, Mysliwiec M. Type of arteriovenous fistule, NHYA class and apelin in hemodialyzed patients. Int Urol Nephrol. 2011;43:185–190
- Lv D, Li H, Chen L. Apelin and APJ, a novel critical factor and therapeutic target for atherosclerosis. Acta Biochim Biophys Sin. 2013;45:527–533
- Kadoglou NP, Sailer N, Moumtzouoglou A, Gerasimidis T, Kostakis A, Liapis CD. Adipokines: a novel link between adioposity and carotid plaque vulnerability. Eur J Clin Invest. 2012;42:1278–1286
- Taşcı I. Apelin, prediabetes and atherosclerosis. Exp Clin Endocrinol Diabetes. 2011;119:457–458