Abstract
Background: The kidney is one of the prior damaged organs subjected to severe infection and sepsis shock. Our previous studies have shown that the normal mesenteric lymph (NML) obtained from healthy dogs could alleviate multiple organ injuries following endotoxic shock. In the current study, we further investigated the beneficial effect of NML from healthy mice on acute kidney injury (AKI) induced by lipopolysaccharide (LPS) in mice. Methods: The mice in LPS and LPS + NML groups received an intraperitoneal injection of LPS (35 mg/kg). One hour later, the treatment of NML was performed and kept for 6 h. Then, the renal function indices, renal morphology, the levels of phosphorylation mitogen-activated protein kinases (MAPKs), markers of sensitization to LPS, as well as pro-inflammatory mediators in renal tissue were observed. Results: Intraperitoneal injection of LPS induced an increased level of urea in plasma, lipopolysaccharide-binding protein (LBP), cluster of differentiation 14 (CD14), tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6), but no obvious changes in the MAPKs in renal tissue. NML treatment decreased the levels of urea, CD14, TNF-α and IL-6 in mice after LPS injection. Conclusion: The current results indicate that NML alleviates LPS-induced AKI through its attenuation of sensitization to LPS.
Introduction
Acute renal failure (ARF) severely worsens prognosis of hospitalized patients, and the combination of ARF and severe sepsis is associated with 70% mortality while mortality rate in patients with non-septic ARF alone is 45%.Citation1,Citation2 To improve the efficiency of early diagnosis and treatment, clinicians recommend the monitoring of the quality of acute dialysis, in which ARF is replaced with acute kidney injury (AKI), including mild acute renal insufficiency.Citation3 Previous studies have shown that the treatment of exogenous normal mesenteric lymph (NML) obtained from healthy dog alleviates the AKI induced by disseminated intravascular coagulation through the improvement of the coagulation function.Citation4 This treatment also protects against AKI caused by intravenous injection of lipopolysaccharide (LPS) through decreasing the leukocyte adhesion and improving the perfusion of kidney.Citation5,Citation6 These results indicate that the NML plays an important beneficial role against AKI.
Increasing studies have demonstrated that mitogen-activated protein kinases (MAPKs) play an important role in the process of systemic inflammatory response syndrome (SIRS) and AKI induced by ischemia-reperfusion, trauma, sepsis, burn, etc.Citation7–9 However, whether the effect of NML on LPS-induced AKI is related to MAPK signaling molecules remains unknown. It has been shown that increased expression of LPS-binding protein (LBP) and activation of cluster of differentiation 14 (CD14), a LPS receptor can enhance the sensitization of inflammatory cells to the endotoxin or LPS that links the inflammatory response to organ injuries.Citation10–12 Wang et al.Citation13 reported that the LBP and CD14 mRNA levels were significantly increased in renal tissue in a mouse model of cecal ligation and puncture-induced sepsis. Nevertheless, it is important to determine whether the LBP and CD14 are upregulated during the LPS-induced AKI. Therefore, the current study was sought to determine the effectiveness of NML on AKI induced by LPS, and the involvement of LBP, CD14 and MAPKs in this process.
Methods
Animals
Thirty-six healthy and specific pathogen free (SPF) BALC/c mice, weighing 20 g–25 g, were purchased from the National Institutes for Food and Drug Control (Beijing, China). Eighteen mice were used to NML drainage for the intervention of AKI induced by LPS, while the rest 18 mice were randomly divided into three groups: sham group, LPS group, and LPS + NML group (n = 6 mice/group). Before the experimentation, the mice were fasted for 12 h, but were allowed free access to water. During the experimentation, all efforts were made to minimize suffering of animals, and the animal experimental procedures were reviewed and approved by the Hebei North University Animal Care Committee and conformed to National Institutes of Health guidelines.
Preparation of NML
All of the mice were anesthetized with intraperitoneal injection of pentobarbital sodium (1%, 50 mg/kg). Then, under the operating microscope (SSJ, Shanghai Optical Instrument Factory, Shanghai, China), a laparotomy with 2 cm longitudinal incision on deviation to right of midline was performed and their mesenteric lymph was drained with a drainage needle continuously for 60 min into a sterile test tube. Lymphatic samples were centrifuged for 5 min at 315 g to remove all cellular components and stored at −75 °C until further experimentation.
Administration of LPS and treatment of NML
After anesthetization with pentobarbital sodium, the femoral operation was performed under the operating microscope. First, the right femoral artery was separated and cannulated for anticoagulation with heparin sodium (1%, 1 mL/kg). Tiny heparinized polyethylene catheter was inserted into the right femoral artery for continuously monitoring the animals' mean artery pressure (MAP) using the RM-6240B biological signal collecting and processing system (Chengdu Inc., Chengdu, China). Secondly, after a 30-min stabilization period, the lipopolysaccharide (LPS, 0.5%, 35 mg/kg) (Escherichia coli O111:B4) (Sigma, Milwaukee, WI) was injected through abdomen in the LPS and LPS + NML groups. Finally, after 60 min of LPS injection, the administration of NML (1/15 of whole blood volume) was performed through femoral artery as described in the previous reportsCitation14,Citation15 in the LPS + NML group. In the sham group, the mice were anesthetized, cannulated and operated as described above, but without LPS injection and NML intervention. And the same amount of saline instead of NML was given to a separate LPS group and sham group as control.
Measurement of renal function
At 6 h after intraperitoneal injection of LPS in the LPS and LPS + NML groups or at corresponding time point in the sham group, blood samples were obtained from the heart through apical centesis, and the plasma was prepared by centrifugation at 850g for 10 min and was stored at −80 °C in refrigerator (Thermo Electron, Waltham, MA) for the further determination of renal function. Levels of Urea and cystatin C (CyC) in plasma were examined by an automatic biochemical analyzer (7600-110, Hitachi, Tokyo, Japan), and the kit was purchased from Randox Laboratories Ltd. (Shanghai, China).
Observation of renal morphology
At 6 h after LPS injection, the left kidney was obtained from each mouse under deeply anesthetic conditions. Subsequently, the kidney was split by a longitudinal midline incision into halves, including medulla and cortex, and then fixed in neutral buffered paraformaldehyde of 4%, dehydrated with alcohol gradient, embedded in paraffin, and sectioned at 4 µm using a microtome (Shandon Finesse 325, Thermo, Waltham, MA). After hematoxylin and eosin (HE) staining, morphological alterations were observed with light microscopy (90-i; Nikon, Tokyo, Japan) and images were collected and their photos were taken using an image collection and analysis system (Motic Med 6.0; Beijing, China). All morphological examinations were conducted by a forensic pathologist without prior knowledge of the experimental conditions.
Preparation of renal homogenate
Similarly, the right kidney was obtained from each mouse at 6 h after LPS administration, was homogenized in 1:9 (w/v) normal saline for 30 s and centrifuged at 850g at 0–4 °C for 10 min using Labofuge 400R super centrifuge (Thermo Fisher Scientific, San Jose, CA), while the supernatant fluid was frozen at −80 °C in refrigerator for next assays.
Measurement of MAPKs
Phosphorylation levels of p38 MAPK, extracellular regulated protein kinases (ERK) 1/2 and c-Jun NH2-terminal protein kinase (JNK) in renal homogenates were determined by the mouse – specific enzyme-linked immunoadsorbent assay (ELISA) kit (antibodies were purchased from R&D Systems, Minneapolis, MN) using the SpectraMax® M3 Microplate Reader (Molecular Devices, Sunnyvale, CA), in accordance with manufacturer's protocols. The standard curves of p-p38 MAPK, p-ERK1/2, and p-JNK were y = 0.008x + 0.0000149x2 0.000000079x3, r2 = 0.9987; y = 0.006x − 0.000070x2 − 0.00000000442x3, r2 = 0.9966; y = 0.004x − 0.0000022x2 + 0.000000000276x3, r2 = 0.9979; respectively. Protein concentration in homogenate was quantified by the Coomassie brilliant blue colorimetric method (kit purchased from Jiancheng Biotechnology Research Institute, Nanjing, China), and results were normalized by protein levels.
Measurement of markers of sensitization to LPS
The levels of LBP and CD14 in renal homogenates were detected using the ELISA kit as described above. And the standard curves of LBP and CD14 were y = 0.128x + 0.003x2 − 0.0001874x3, r2 = 0.9992; y = 0.061x + 0.00004486x2 − 0.000004574x3, r2 = 0.9995; respectively.
Measurement of pro-inflammatory mediators
Meanwhile, the contents of TNF-α and IL-6 in renal homogenates were determined with the method of ELISA after manufacturing standard curves (y = 0.016x − 0.0004087x2 + 0.00000006004x3, r2 = 0.9977; y = 0.045x − 0.00005297x2 − 0.000003114x3, r2 = 0.9993; respectively).
Statistical analysis
Data were presented as mean ± SD. Statistical analysis was performed using SPSS 19.0 (Polar Engineering and Consulting Inc., Chicago, IL). The one-way ANOVA was conducted between groups and Student–Newman–Keuls test was used within groups. p < 0.05 was considered to be statistically significant.
Results
Renal function indices in plasma
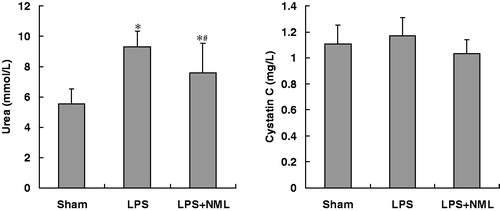
At 6 h after LPS injection, the levels of plasma urea in both LPS and LPS+NML groups were significantly increased when compared to that of sham group. However, the plasma urea content in LPS+NML was lower than that in the LPS group (p < 0.05). There were no statistical differences in CyC levels among the three groups (p > 0.05, ).
Renal morphology
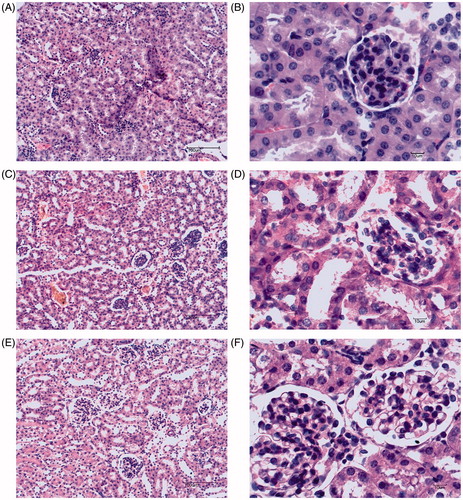
In the sham group, there was normal glomerular and tubular structures, neatly arranged tubular epithelial cells, and clear proximal and distal convoluted tubule in kidney of mice (, B). In contrast, a little extravasated blood in renal interstitium appeared in kidney of the LPS administrated mice (, D). There was no obvious abnormality in the structure of renal glomerulus and tubules in the LPS + NML group (, F).
MAPKs in renal tissue
At 6 h after LPS injection, there were no statistical differences in p-MAPK, p-ERK 1/2, and p-JNK in renal homogenate among the sham, LPS, and LPS + NML groups (p > 0.05) (see ).
Table 1. Effects of NML on the phosphorylation levels of p38 MAPK, ERK 1/2, and c-Jun NH2-terminal protein kinase (JNK) in kidney after LPS administration in mice (ng/mg protein, mean±SD, n = 6).
Sensitization to LPS in renal tissue
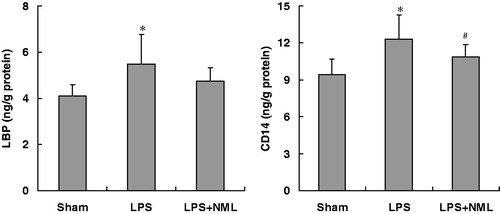
As shown in , LPS administration significantly increased the LBP and CD14 levels of renal homogenate (p < 0.05). The NML treatment intended to decrease the level of LBP, but did not reach to statistical differences (p > 0.05). In contrast, the NML treatment reversed the increase in CD14 content in renal tissue produced by LPS injection (p < 0.05).
Pro-inflammatory mediators in renal tissue
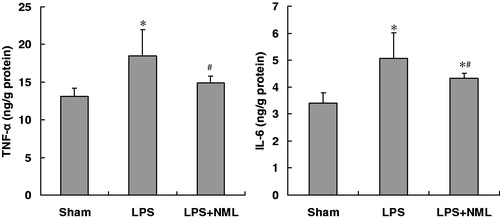
At 6 h after LPS intraperitoneal injection, the levels of TNF-α and IL-6 in renal homogenate in the LPS group were significantly increased compared to that of the sham group (p < 0.05, ). The NML treatment resulted in a significant decrease in the TNF-α and IL-6 contents in renal tissue (p < 0.05).
Discussion
In the present study, we established the mouse model with LPS attack through intraperitoneal injection. We found that the AKI came on in mice at 6 h after LPS administration, which was presented with a little extravasated blood in renal tissue and increased urea in plasma. Moreover, we harvested NML from healthy mice for the intervention of LPS-induced AKI, and found that NML treatment could significantly attenuate LPS-induced renal tissue damage and increase in urea in the plasma. Besides, in our previous report related with this study, we found that the MAP in the LPS group at the 90 min ∼ 360 min (excepted for 100 min) was remarkably decreased than that of the sham group; at the 80 min, 90 min, 190 min, 210 min, 240 min, 250 min, 340 min, 350 min, and 360 min, NML treatment significantly increased the levels of MAP in mice following LPS administration.Citation16 These results suggested that NML plays a protective effect against AKI caused by LPS intraperitoneal injection.
Previous studies have shown that the elevated CyC level in serum or urine is one of the good indicators of acute renal damage due to I/R produced by renal artery occlusion,Citation17 adult cardiothoracic surgery,Citation18,Citation19 contrast,Citation20,Citation21 etc. In the present study, we also measured the level of CyC in plasma of mice. However, we found no changes in CyC content in plasma in either LPS group or NML-treated group. The main reason might be the short observation time in this current study, which was 6 h after LPS injection. Meanwhile, renal histomorphology observation in this study showed that the structure of glomerulus in the LPS group was basic normal, which indicated the degree of kidney injury was mild. Because CyC level is associated with glomerular structure and filter function, the unaltered CyC level may be due to the short observation time or to the mild kidney injury.
It is generally accepted that SIRS is associated with multiple organ injuries,Citation22 and that LPS is a powerful tool to induce SIRS with AKI.Citation23,Citation24 LPS triggers macrophage activation through binding to the transmembrane signaling receptor toll-like receptor 4, which forms an active complex with LBP, CD14 and myeloid differentiation protein 2.Citation25,Citation26 Furthermore, the complex induces the produce and release of inflammatory mediators, lead to SIRS. Therefore, we investigated primarily the changes of LBP and CD14 in renal tissue, and found that intraperitoneal injection of LPS with a dose of 35 mg/kg significantly increased the levels of LBP and CD14 in renal tissue, while the CD14 level was markedly decreased by the NML treatment. Therefore, the role of NML on AKI is correlative with CD14, an important marker of sensitization to LPS. Nevertheless, the character of LBP during NML alleviating LPS-induced AKI is required to be studied in the future.
TNF-α and IL-6 are the most important cytokines following LPS challenge, and the levels of TNF-α and IL-6 can reflect the degree of inflammatory response.Citation27 Therefore, the current study examined the changes of TNF-α and IL-6 in renal tissue after LPS challenge, these results showed that LPS intraperitoneal injection induced obvious increases in the TNF-α and IL-6 levels of renal tissue. These deleterious effects of LPS administration on pro-inflammatory mediators were significantly ameliorated by NML treatment. Therefore, the role of NML alleviating LPS-induced AKI is related to lessening the levels of pro-inflammatory mediators.
During the LPS stimulation leading to inflammatory response, the several intracellular signaling pathways, including the MAPK pathway and IkappaB kinase-nuclear factor kappa B (NF-κB) pathway, coordinate the induction of many genes encoding inflammatory mediators.Citation28 The MAPK signal components, including p38 MAPK, JNK, and ERK, are involved in modulating the critical inflammatory response. After stimulation with LPS, p38 MAPK promotes transcription of TNF-α and IL-1β, JNK regulates the production of many cytokine, such as TNF-α, IL-6, and type I IFNs. Although not affecting cytokine transcription, ERK modulates the stabilization of TNF-α mRNA in LPS-treated macrophages.Citation29,Citation30
Hence, we determined the phosphorylation levels of p38 MAPK, ERK1/2, and JNK in renal tissue. But, the results showed that there was no difference among three groups. Its reason may be related to the short observation time and the dose of LPS injection. In our previous study, we found that LPS induced the increase in p38 MAPK, ERK1/2, and JNK in pulmonary tissue, and NML administration reduced the phosphorylation levels of MAPKs.Citation16 Thus, we thought that it was also related to the different organ. So, the role of MAPKs on the LPS-induced AKI needs further research. In addition, NF-κB is the other main signal pathway, which induces the expression of pro-inflammatory mediators. Therefore, whether NML decreasing the inflammatory mediators' levels in kidney is related to NF-κB signal pathway, needs further investigation in the future.
In summary, the current study established the method of NML drainage from mouse and demonstrated the therapeutic effect of mouse NML on LPS-induced AKI. The results indicate that the beneficial effects of small amount NML on the AKI caused by LPS is related to lessening the sensitization to LPS and to decreasing the cytokine production.
Declaration of interest
No benefits in any form have been received or will be received from a commercial association related directly or indirectly to the subject of this article. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by the scientific research projects of Education Department in Hebei Province (2005311, 2007407).
References
- Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112(4):460–467
- Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169
- Bellomo R, Ronco C, Kellum JA, et al. Acute dialysis quality initiative workgroup. acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs. Crit Care. 2004;8(4):204–212
- Zhao ZG, Niu CY, Zhang YP, et al. Exogenous normal lymph alleviating kidney injury by improving coagulation function in disseminated intravascular coagulation rats. Ren Fail. 2012;34(2):221–226
- Zhao ZG, Niu CY, Zhang LL, et al. Exogenous normal lymph alleviates lipopolysaccharide-induced acute kidney injury in rats. Ren Fail. 2013;35(6):806–811
- Zhang LL, Zhang J, Zhao ZG, et al. Intervention role of lymph plasma on renal and hepatic blood perfusion in rats with endotoxic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22(12):740–743
- Chen J, Wang W, Zhang Q, et al. Low molecular weight fucoidan against renal ischemia-reperfusion injury via inhibition of the MAPK signaling pathway. PLoS One. 2013;8(2):e56224
- Rossaint J, Spelten O, Kässens N, et al. Acute loss of renal function attenuates slow leukocyte rolling and transmigration by interfering with intracellular signaling. Kidney Int. 2011;80(5):493–503
- Feng Y, Liu Y, Wang L, et al. Sustained oxidative stress causes late acute renal failure via duplex regulation on p38 MAPK and Akt phosphorylation in severely burned rats. PLoS One. 2013;8(1):e54593
- Fang WH, Yao YM, Shi ZG, et al. Effect of recombinant bactericidal/permeability-increasing protein on endotoxin translocation and lipopolysaccharide-binding protein/CD14 expression in rats after thermal injury. Crit Care Med. 2001;29(7):1452–1459
- Fang H, Liu A, Dirsch O, et al. Liver transplantation and inflammation: Is lipopolysaccharide binding protein the link? Cytokine. 2013;64(1):71–78
- Fang H, Liu A, Sun J, et al. Granulocyte colony stimulating factor induces lipopolysaccharide (LPS) sensitization via upregulation of LPS binding protein in rat. PLoS One. 2013;8(2):e56654
- Wang SC, Klein RD, Wahl WL, et al. Tissue coexpression of LBP and CD14 mRNA in a mouse model of sepsis. J Surg Res. 1998;76(1):67–73
- Li Y, Liu B, Fukudome EY, et al. Identification of citrullinated histone H3 as a potential serum protein biomarker in a lethal model of lipopolysaccharide-induced shock. Surgery. 2011;150(3):442–451
- Liu C, Zhang GF, Song SW, et al. Effects of ketanserin on endotoxic shock and baroreflex function in rodents. J Infect Dis. 2011;204(10):1605–1612
- Du HB, Song W, Zhang LM, et al. Effect of normal lymph on lung, heart and liver injuries in mice with endotoxic shock. Zhongguo Bing Li Sheng Li Zazhi. 2014;30(4):686–692
- Sahsivar MO, Narin C, Kiyici A, et al. The effect of iloprost on renal dysfunction after renal I/R using cystatin C and beta2-microglobulin monitoring. Shock. 2009;32(5):498–502
- Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74(8):1059–1069
- Che M, Xie B, Xue S, et al. Clinical usefulness of novel biomarkers for the detection of acute kidney injury following elective cardiac surgery. Nephron Clin Pract. 2010;115(1):c66–c72
- Yin L, Li G, Liu T, et al. Probucol for the prevention of cystatin C-based contrast-induced acute kidney injury following primary or urgent angioplasty: A randomized, controlled trial. Int J Cardiol. 2013;167(2):426–429
- Briguori C, Visconti G, Rivera NV, et al. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121(19):2117–2122
- Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107
- Takahashi K, Mizukami H, Kamata K, et al. Amelioration of acute kidney injury in lipopolysaccharide-induced systemic inflammatory response syndrome by an aldose reductase inhibitor, fidarestat. PLoS One. 2012;7(1):e30134
- Han M, Li Y, Liu M, et al. Renal neutrophil gelatinase associated lipocalin expression in lipopolysaccharide-induced acute kidney injury in the rat. BMC Nephrol. 2012;13:25
- Viladés C, Escoté X, López-Dupla M, et al. Involvement of the LPS-LPB-CD14-MD2-TLR4 inflammation pathway in HIV-1/HAART-associated lipodystrophy syndrome (HALS). J Antimicrob Chemother. 2014;69(6):1653–1659
- Fossum C, Hjertner B, Olofsson KM, et al. Expression of tlr4, md2 and cd14 in equine blood leukocytes during endotoxin infusion and in intestinal tissues from healthy horses. Vet Immunol Immunopathol. 2012;150(3–4):141–148
- Myers MJ, Farrell DE, Palmer DC, et al. Inflammatory mediator production in swine following endotoxin challenge with or without co-administration of dexamethasone. Int Immunopharmacol. 2003;3(4):571–579
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94
- Wang T, Wu F, Jin Z, et al. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem Toxicol. 2014;64:177–183
- Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90(4):1507–1546