Abstract
Introduction: Urine neutrophil gelatinase-associated lipocalin (uNGAL) is a rapidly emerging biomarker for early detection of acute kidney injury (AKI). We aimed to investigate the prevalence and prognostic value of the early uNGAL in patients with AKI induced by sepsis. Methods: In this prospective cohort study, we analyzed the case records of 126 septic patients with and without AKI and evaluated the uNGAL for early prediction and risk stratification of septic patients with AKI. Results: Of 126 patients analyzed, 58 (46%) developed septic AKI. Men comprised more than half (68%) of the sample population, the mean age (SD) was 57 years. The prognostic accuracy of uNGAL, as quantified by the area under the receiver-operating-characteristic curve (AU-ROC), was highest with peak uNGAL (AU-ROC: 0.86; 95% CI: 0.81–0.93), as compared with the admission uNGAL (AU-ROC: 0.81; 95% CI: 0.73–0.89). The peak uNGAL correlated with the levels of peak blood urea nitrogen (r = 0.674) and serum creatinine (r = 0.608), the length of hospital stay (r = 0.602) and weakly correlated with the number of hemodialysis sessions that each patient received during hospital stay (r = 0.405). By multivariate analysis, increased peak uNGAL remained independently associated with the development of septic AKI (odds ratio: 32.12; 95% CI: 6.21–90.37; p < 0.0001). Conclusions: uNGAL is independently associated with subsequent AKI among patients with sepsis.
Introduction
Sepsis is the most common trigger of acute kidney injury (AKI) in critically ill patients. A prospective observational study described that sepsis was recognized as the most important contributing factor for AKI, and the rate of approximately 50%.Citation1 Other studies reported that 40%–75% of AKI were associated with sepsis.Citation2–4 These patients generally had a poorer prognosis when compared to septic non-AKI.Citation5–7 Therefore, early detection of septic AKI patients is of great importance to enable adequate treatment in these patients and improve their outcomes.
The experimental reports have suggested that there may be important pathophysiologic differences between septic and septic non-AKI.Citation8,Citation9 Considering these differences, discriminating septic and septic non-AKI may have clinical prognostic importance. In current clinical practice, AKI is typically diagnosed by measuring blood urea nitrogen (BUN) and serum creatinine (SCr), but it is well recognized that BUN and SCr are insensitive and late indicators of AKI.Citation10 More recently, urine neutrophil gelatinase-associated lipocalin (uNGAL) has been considered more sensitive and specific test to detect AKI.Citation11 However, uNGAL is also a marker of systemic inflammation since it is typically released by neutrophils upon activation.Citation12 So, it is extremely important to make clear the relationship between uNGAL and septic AKI.
The main objective of this study was to estimate the diagnostic accuracy of uNGAL in general intensive care unit (ICU) for the early detection of septic AKI. Additional objectives were to explore the relationship between uNGAL, development of severe AKI and the length of hospital stay, as well as to evaluate uNGAL as a biomarker for continuous renal replacement therapy (CRRT) use and mortality in the ICU.
Methods
Study population
We collected the case records of 126 patients with sepsis from May 2012 to March 2014 at the Ningbo First Hospital of the Ningbo University in Ningbo. Patients were included if they fulfilled all the following criteria: (1) adult (age ≥18 years); (2) patients admitted to ICU; (3) evidence of sepsis;Citation13 and (4) the patients who did not have any of the exclusion criteria. Patients with end-stage kidney disease, defined as estimated glomerular filtration rate <15 mL/min/m2, chronic dialysis therapy, confirmed and/or suspected acute glomerulonephritis, acute interstitial nephritis, renal vasculitis or postrenal etiology for AKI and who had just undergone renal transplantation or prior kidney transplant were excluded. The study was approved by the ethics committees of Ningbo First Hospital. An informed consent was obtained either from patients or their families.
Definitions
AKI was defined according to the risk of renal dysfunction, injury to the kidney, failure of kidney function, loss of kidney function and end-stay kidney disease (RIFLE) classification scheme.Citation14 Patients were diagnosed and severity classified based on changes from baseline SCr and/or changes in urine output. Baseline SCr was defined by the lowest outpatient SCr in the six months preceding index hospitalization. II score. Oliguria was defined as a urine output <30 mL/h. Sepsis syndrome was defined according to consensus guidelines.Citation15
Clinical data collection
Clinical and laboratory data were collected on standardized data collection forms. Data requested from participating patients included patient age, sex, height and weight; admission and discharge diagnoses; the value of uNGAL [Urine were collected twice daily (6 am and pm generally) until discharge or earlier if hemodialysis was initiated, uNGAL was measured by the polyclonal antibody-based radioimmune assays (RIA)],Citation16 BUN, SCr and uric acid; the length of hospital stay, the need for hemodialysis treatment and the number of hemodialysis sessions; the presence of sepsis, proteinuria, hematuria and severe complications. All subjects were admitted to the hospital and monitored daily until discharged. CRRT treatment was guided by the physician based upon clinical necessity.
Statistical analysis
Continuous variables were presented as medians with the interquartile range (IQR) and categorical variables as numbers and percentages. Continuous variables were compared with the use of the nonparametric Mann–Whitney U test and categorical variables with the use of Fisher’s exact test or the Pearson’s χ2 test. Spearman correlations and the linear regression analyses were used to evaluate the relationship between the early uNGAL and the later biochemical or clinical parameters. Receiver-operating-characteristic (ROC) curves were constructed to assess the sensitivity and specificity of uNGAL in predicting the development of septic AKI and the need of hemodialysis. Multivariate logistic regression model was used to identify uNGAL associated with the development of septic AKI. Covariates entered into the model included age, gender and the presence of sepsis, proteinuria and hematuria preceding the development of septic AKI. All analyses were performed using Statistical Package for Social, version 18.0 (SPSS Inc., Chicago, IL). A two-tail p value <0.05 was interpreted as significant.
Results
Characteristics of the patients
In total, 126 patients were enrolled, 58 (46%) with septic AKI. Of these patients, 19 females and 39 males were admitted to the hospital seven days (IQR 6–8 days) after acute onset of clinical symptoms. During their stay in ICU, “risk” for renal failure was developed in 58 (46%) patients at four days (IQR 3–5 days) of their ICU admissions; “injury” (AKI) developed in 47 (37%) patients at 12 days (IQR 10–14 days) of their ICU admissions and “failure” developed in 26 (21%) patients at 16 days (IQR 13–19 days) of their ICU admissions. Hemodialysis was performed in 23 (40%) patients.
The baseline information of patients, laboratory test results and ICU outcomes were compared and summarized in . A remarkable result presented in this table is that the length of hospital and ICU mortality [the overall mortality was 59 (47%)] were significantly higher in “septic AKI group” when compared with the “sepsis non-AKI group” (p < 0.0001). Patients developing septic AKI were older, and more likely to have hematuria, proteinuria and therefore need acute hemodialysis than patients who had septic non-AKI.
Table 1. Characteristics of patients by septic AKI status.
Association of uNGAL with renal function
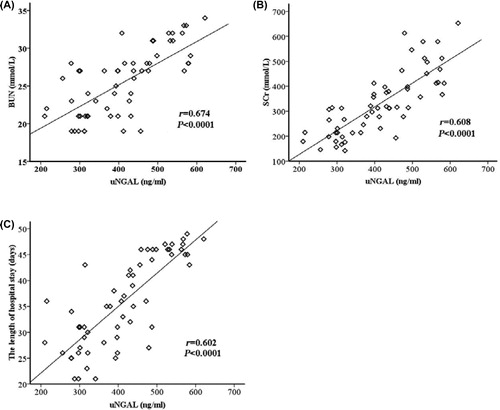
Urine NGAL is an early, common and consistent laboratory finding in patients with sepsis. The early increasing uNGAL reached their peak seven days (IQR 6–8 days) after the acute onset of sepsis, followed by consistent rises in the levels of BUN and SCr in patients developing septic AKI reaching their maximums 10 days (IQR 8–12 days) and 11 days (IQR 9–14 days), respectively. In these patients, uNGAL preceded the peaks of BUN and SCr by three days (2–4 days) and four days (2–6 days), respectively. The peak uNGAL correlated positively with the peak BUN (r = 0.674, p < 0.0001), peak SCr (r = 0.608, p < 0.0001) and the length of hospital stay (r = 0.602, p < 0.0001) (). Significant but weaker correlations were found between peak uNGAL and peak uric acid (r = 0.340, p = 0.009), hematuria (r = 0.173, p < 0.0001) and proteinuria (r = 0.209, p < 0.0001).
Association of uNGAL with septic AKI development
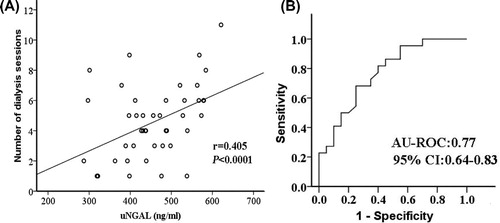
The admission and peak uNGAL were significantly higher in patients with septic AKI than those with septic non-AKI (). The accuracy for the prediction of septic AKI, as quantified by the area under the receiver-operating-characteristic curve (AU-ROC), was significantly higher with the peak uNGAL than that with the admission uNGAL (AU-ROC for the peak uNGAL: 0.86; 95% CI: 0.81–0.93; vs. AU-ROC for the admission uNGAL: 0.81; 95% CI: 0.73–0.89; p = 0.039) (). At a cut-off value of 402 ng/mL, the sensitivity and specificity to predict septic AKI were 89% and 74% for the peak uNGAL, respectively. After adjustment for age, gender and for other variables preceding the development of septic AKI, increased peak uNGAL (odds ratio: 32.12; 95% CI: 6.21–90.37; p < 0.0001) and the presence of hematuria (odds ratio: 7.63; 95% CI: 3.21–35.67; p < 0.001) remained independent predictors of septic AKI ().
Figure 2. Receiver-operating-characteristic (ROC) curves for uNGAL to predict the development of septic AKI.
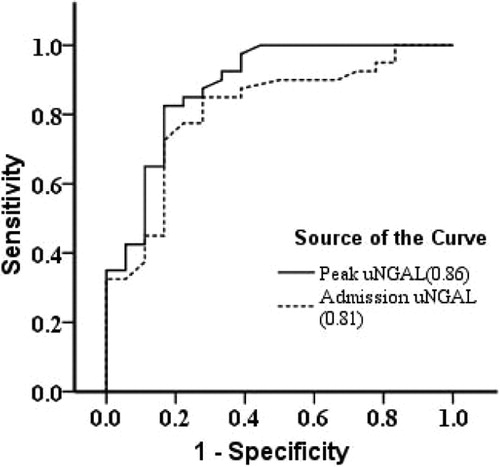
Table 2. Multivariable logistic regression for the prediction of septic AKI combining peak uNGAL with other variables.
Association of uNGAL with hemodialysis treatment
Of 58 patients, 23 (40%) received one or more sessions of CRRT treatment. We observed a weak correlation between the peak uNGAL and the number of hemodialysis sessions that each patient received during hospital stay (r = 0.405, p < 0.0001) (). The peak uNGAL were significantly higher in patients receiving hemodialysis compared with those not receiving hemodialysis (median, 456 ng/mL vs. 341 ng/mL; p < 0.0001). However, there were no differences between the peak uNGAL in patients who received one or more than one hemodialysis session. The AU-ROC for the peak uNGAL in the prediction of hemodialysis was 0.77 (95% CI: 0.64–0.83) (). At a cut-off level of 494 ng/mL, the sensitivity and specificity of the peak uNGAL in predicting hemodialysis were 89% and 71%, respectively.
Discussion
In this study, the clinical processes of patients with sepsis were prospectively followed throughout hospitalization. AKI is an important cause of morbidity and mortality in ICU.Citation17–19 Our study remarkably once again pointed out this fact as the length of hospital stay and mortality was significantly higher in “septic AKI group” when compared with “septic non-AKI group”. This result underlines the importance of earlier detection of septic AKI, especially in emergency departments and ICU.
During the course of sepsis, the patients sequentially developed mild-to-severe AKI. In these patients, the peak uNGAL positively correlated with the levels of BUN, SCr and the number of hemodialysis sessions, reflecting the severity of renal dysfunction. The peak uNGAL in patients with sepsis was strongly associated with a substantially increased risk for the development of septic AKI.
Little is known about the prognostic importance of uNGAL in patients with sepsis. Approximately 65% of the patients in the study of sepsis had increased uNGAL. These patients also had a higher level of BUN, SCr and prevalence of AKI.Citation20 By contrast, the uNGAL was also measured in one other study of sepsis in which 63 (42%) of 151 patients had increased uNGAL.Citation21 Similarly, a recent study in a cohort of 45 sepsis patients showed the parameter uNGAL was a good diagnostic marker for AKI development, but not for AKI severity, when it was recorded after a rise in SCr had occurred.Citation22–24
In our study, the peak uNGAL was found to be correlated with the laboratory and clinical parameters, reflecting the severity of septic AKI (). The diagnostic accuracy for septic AKI was highest with the peak uNGAL, as compared with the admission uNGAL (). Similar to the previous uNGAL study,Citation14,Citation25 the presence of hematuria preceding the development of septic AKI was also an independent predictor (). We further observed that the peak uNGAL was weakly correlated with the number of hemodialysis sessions each patient received during hospital stay, and ROC curve yielded an AU-ROC of 0.77 for predicting hemodialysis ().
With the earlier detection of septic AKI, earlier interventions would be made such as early antibiotic initiation, fluid resuscitation and restricting nephrotoxic antibiotic and intravenous contrast dye use. In order to diagnose AKI earlier than SCr, follow up of uNGAL levels might be used in ICU. Our result pointed out that follow up of uNGAL was specific for the diagnosis of AKI with an AU-ROC of 0.86. Among the patients with septic AKI, timely initiation of hemodialysis may reduce subsequent complications and death risk.Citation26 The lever of uNGAL may make it possible both to rule in and rule out the prognosis of septic AKI and need of hemodialysis on the basis of the daily measurements.
There are several limitations to our study. First, this study was relatively small and prospective in nature, our findings may be prone to a type II error, therefore, these markers require validation in independent cohorts. Second, this study cannot differentiate the peak levels of BUN and SCr between patients with and without acute hemodialysis treatment. The values were distinctly underestimated in patients who had undergone hemodialysis; the coefficient was thereby under ascertained. Other early abnormalities, such as hypoalbuminemia, elevated hematocrit and elevated aspartate aminotransferase and alanine aminotransferase indicating the presence of hemoconcentration and liver impairment, were also noted in sepsis, but were not included in the analysis because they are not measured routinely in patients with sepsis.
Conclusions
The early increased uNGAL was associated with the development of septic AKI, length of hospital stay, need of hemodialysis and mortality. The uNGAL is a simple and readily available test that is widely used in medical care at present. The increased uNGAL significantly will be an early and consistent laboratory abnormality in septic AKI, it could be of value in risk stratification of patients for the timely initiation of hemodialysis treatment. Of potential interest to physicians is whether therapeutic decrease in uNGAL might be useful in the prediction of improvement in clinical severities.
Declaration of interest
The authors declare that they have no competing interests with this scientific work.
References
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294(7):813–818
- Neveu H, Kleinknecht D, Brivet F, et al. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11(2):293–299
- Silvester W, Bellomo R, Cole L. Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med. 2001;29(10):1910–1915
- Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: A population-based study. Crit Care. 2005;9(6):R700–R709
- Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439
- Oppert M, Engel C, Brunkhorst FM, et al. Acute renal failure in patients with severe sepsis and septic shock—A significant independent risk factor for mortality: Results from the German Prevalence Study. Nephrol Dial Transplant. 2008;23(3):904–909
- Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care. 2008;12(2):R47
- Ishikawa K, May CN, Gobe G, et al. Pathophysiology of septic acute kidney injury: A different view of tubular injury. Contrib Nephrol. 2010;165:18–27
- Wan L, Bagshaw SM, Langenberg C, et al. Pathophysiology of septic acute kidney injury: What do we really know? Crit Care Med. 2008;36(4 Suppl):S198–S203
- Abraham BP, Frazier EA, Morrow WR, et al. Cystatin C and neutrophil gelatinase-associated lipocalin as markers of renal function in pediatric heart transplant recipients. Pediatr Transplant. 2011;15(6):564–569
- Kim H, Hur M, Cruz DN, et al. Plasma neutrophil gelatinase-associated lipocalin as a biomarker for acute kidney injury in critically ill patients with suspected sepsis. Clin Biochem. 2013;46(15):1414–1418
- Wheeler DS, Devarajan P, Ma Q, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36(4):1297–1303
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530–538
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13(10):818–829
- Xu SY, Petersson CG, Carlson M, et al. The development of an assay for human neutrophil lipocalin (HNL)-to be used as a specific marker of neutrophil activity in vivo and vitro. J Immunol Methods. 1994;171:245–252
- Clark WR, Letteri JJ, Uchino S, et al. Recent clinical advances in the magagement of critically ill patients with acute renal failure. Blood Purif. 2006;24(5–6):487–498
- Baqshaw SM, Bennett M, Devarajan P, et al. Urine biochemistry in septic and non-septic acute kidney injury: A prospective observational study. J Crit Care. 2013;28(4):371–378
- Guo Y, Yan KP. Prognostic significance of urine neutrophil gelatinase-associated lipocalin in patients with septic acute kidney injury. Exp Ther Med. 2011;2(6):1133–1139
- Martensson J, Bell M, Oldner A, et al. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med. 2010;36(8):1333–1340
- Aydogdu M, Gürsel G, Sancak B, et al. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers. 2013;34(4):237–246
- Zappitelli M, Washburn KK, Arikan AA, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Crit Care. 2007;11(4):R84
- De Geus HR, Woo JG, Wang Y, et al. Urinary neutrophil gelatinase-associated lipocalin measured on admission to the intensive care unit accurately discriminates between sustained and transient acute kidney injury in adult critically ill patients. Nephron Extra. 2011;1(1):9–23
- Smertka M, Wroblewska J, Suchojad A, et al. Serum and urinary NGAL in septic newborns. Biomed Res Int. 2014;2014:717318
- Baqshaw SM, Bennett M, Haase M, et al. Plasma and urine neutrophil gelatinaseassociated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36(3):452–461
- Liu KD, Himmelfarb J, Paganini E, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–919