Abstract
Purpose: Acute renal infarction is often missed or diagnosed late due to its rarity and non-specific clinical manifestations. This study analyzed the clinical and laboratory findings of patients diagnosed with renal infarction to determine whether it affects short- or long-term renal prognosis. Methods: We retrospectively reviewed the medical records of 100 patients diagnosed as acute renal infarction from January 1995 to September 2012 at Gyeongsang National University Hospital, Jinju, South Korea. Results: Acute kidney injury (AKI) occurred in 30 patients. Infarct size was positively correlated with the occurrence of AKI (p = 0.004). Compared with non-AKI patients, AKI occurrence was significantly correlated with degree of proteinuria (p < 0.001) and the presence of microscopic hematuria (p = 0.035). AKI patients had higher levels of aspartate transaminase (p < 0.001), alanine transaminase (p < 0.001), and lactated dehydrogenase (p = 0.027). AKI after acute renal infarction was more common in patients with chronic renal failure (CRF) (eGFR < 60 mL/min), compared with non-CRF patients, whose baseline eGFR was >60 mL/min (p = 0.003). Most patients recovered from AKI, except for seven patients (7%) who developed persistent renal impairment (chronic kidney disease progression) closely correlated with magnitude of infarct size (p = 0.015). Six AKI patients died due to combined comorbidity. Conclusions: AKI is often associated with acute renal infarction. Although most AKI recovers spontaneously, renal impairment following acute renal infarction can persist. Thus, early diagnosis and intervention are needed to preserve renal function.
Introduction
Acute renal infarction results from acute occlusion of the renal arteries and segmental branches attributable to the intrinsic pathology of the renal arteries, abdominal trauma, or embolization of thrombi arising in the heart or proximal aorta.Citation1,Citation2 Thrombosis can occur as a complication of progressive atherosclerosis, which can be an important cause of progressive renal insufficiency. In other patients, thrombosis may be associated with thrombophilic states such as anti-phospholipid antibody syndrome and inflammatory disorders. In situ thrombosis has been observed in structural lesions of the renal arteries, such as fibromuscular dysplasia or renal artery aneurysms. Renal infarction has been associated with medical conditions such as vascular inflammatory diseases, including polyarteritis nodosa, Takayasu's arteritis, Kawasaki's disease, and Behçet's disease; nephrotic syndrome; infectious diseases, including syphilis; and sickle cell anemia.Citation1–3 Renal infarction can also arise from unknown etiology.Citation4
Contrast-enhanced computed tomography (CT) is the imaging modality of choice for evaluating various acute abdominal conditions. Diagnosis of acute renal infarction is often missed or delayed because of the rarity of the disease and its nonspecific clinical manifestations; thus, CT results may be the first indication of unsuspected renal infarction.Citation5,Citation6 Although we suspect that the severity of renal infarction affects short- or long-term renal function, there are no large-scale reports describing this. To evaluate the effect of renal infarction on renal function prognosis, we retrospectively reviewed medical records of patients with renal infarction. We focused on the relationship between clinical, radiologic, and laboratory characteristics of renal infarction and acute kidney injury (AKI) and persistent renal impairment.
Materials and methods
Patients
We retrospectively analyzed the medical records of 100 patients with acute renal infarction admitted to Gyeongsang National University Hospital (Jinju, South Korea) from January 1995 to September 2012. All had documented medical histories and underwent medical review, as well as a routine general physical examination. Clinical and laboratory data were reviewed to evaluate acute renal infarction. We excluded other diseases that could be mistaken for acute abdominal pain through medical history and laboratory and radiologic examinations.
Diagnosis of acute renal infarction
A diagnosis of acute renal infarction was made based on radiologic evidence from CT scans and various clinical symptoms and signs such as abdominal and/or flank pain, anorexia, nausea, vomiting, fever, oliguria, abdominal tenderness, and hypertension, as well as well-established laboratory findings, including lactated dehydrogenase (LDH), alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT), microscopic hematuria, and proteinuria. CT findings for diagnosis of renal infarction included the presence of a wedge-shaped parenchymal perfusion defect with or without a cortical rim sign, and without mass effect or major peri-renal stranding.
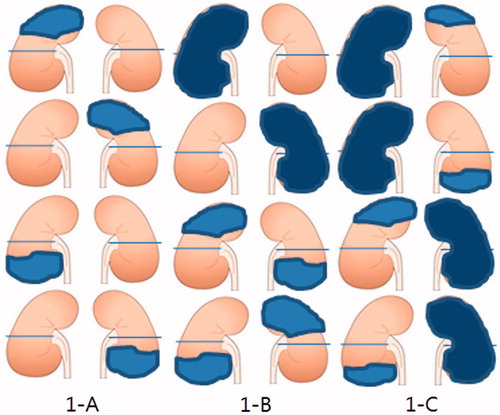
The magnitude of acute renal infarction on CT was divided into three groups according to the sum total of the non-enhanced area on one and/or both kidneys: <25%, 25–50%, and >50%. We assumed that one kidney had 50% capacity, giving 100% capacity for both kidneys. A non-enhanced area of <25% was defined as kidney infarction restricted to one kidney, with a non-enhanced lesion of less than half of one kidney (); 25–50% was defined as non-enhanced lesions restricted to one or two kidneys, with involvement of more than half of one kidney or less than half of each of the two kidneys (); >50% was defined as renal infarction involving more than half of each of the two kidneys ().
Figure 1. Magnitude of renal infarction. Shaded area was noted as infarction area. 1-A (less than 25%); 1-B (25–50%); 1-C (more than 50%).
AKI was defined as a serum creatinine level that increased by more than 0.3 mg/dL or 150% over baseline level within 48 h and the sudden impairment of kidney function resulting in the retention of nitrogenous and other waste products normally cleared by the kidneys.Citation7 Hypertension was defined as blood pressure >140/90 mmHg. Proteinuria was diagnosed by a dipstick test, and hematuria was diagnosed when more than 3–5 red blood cells/HPF were present in the urine. The estimated glomerular filtration rate (eGFR) was calculated from baseline serum creatinine using the four-variable Modification of Diet in Renal Disease Study (MDRD) formula.Citation8 Chronic renal failure (CRF) was defined as eGFR <60 mL/min. Chronic kidney disease (CKD) progression was diagnosed when the serum creatinine level did not return to baseline and persisted at 0.3 mg/dL or 150% over baseline for more than three months. The maximal laboratory values were defined as the peak levels of each value during hospitalization and were used to compare clinical and/or laboratory findings according to the magnitude of renal infarction, AKI, and CKD progression. To evaluate whether acute renal infarction affects AKI or CKD progression, we divided the patients into two groups: the AKI and non-AKI groups, or the CKD progression and non-CKD progression groups, respectively. The study protocol was approved by the Institutional Review Board (IRB No: 2013-02-010).
Statistical analysis
All measurement values are expressed as means ± standard deviation (SD). We used Pearson's chi-square test to analyze qualitative differences. The parametric Student's t-test was performed for the comparison of means in samples of similar variance. One-way analysis of variance was used for comparison of means in three groups. p Value of less than 0.05 was taken to indicate statistical significance. Statistical analysis was carried out using a commercially available statistical package, SPSS for windows version 14.0 (SPSS, Chicago, IL).
Results
Clinical and laboratory characteristics
Atrial fibrillation was most common among the underlying conditions of the patients (). The primary symptoms on admission included abdominal or flank pain (91%), nausea (29%), vomiting (22%), anorexia (14%), and oliguria (1%). The initial physical examinations revealed abdominal tenderness (74%), costovertebral angle (CVA) tenderness (54%), hypertension (35%), and fever (10%). The initial average laboratory values were as follows: ALP, 90.6 ± 4.8 U/L; AST, 90.1 ± 12.1 U/L; ALT, 52.6 ± 4.8 U/L; and LDH, 629.8 ± 45.9. The maximal values of these parameters during hospitalization were ALP, 112.8 ± 6.8 U/L; AST, 114.2 ± 19.9 U/L; ALT, 89.7 ± 7.9 U/L; and LDH, 807.2 ± 63.6. The frequencies of abnormal values during hospitalization were ALP, 9%; AST, 61%; ALT, 50%; and LDH, 96.4% (). The mean blood urea nitrogen (BUN) and serum creatinine values at the time of admission were 20.5 ± 10.8 mg/dL and 1.16 ± 0.68 mg/dL, respectively. The incidences of hematuria and proteinuria were 60% and 56% (trace 33%, +17%, ++6%), respectively. Forty patients had renal infarction on the right side; 40, on the left side; and 20, in both kidneys. Fifty-two patients had <25% renal infarction, 31 had 25–50% renal infarction, and 17 had >50% renal infarction.
Table 1. Baseline characteristics, underlying disease, and clinical manifestations of patients with renal infarction.
Table 2. Initial and maximal laboratory values in the patients with renal infarction.
Laboratory findings according to magnitude of renal function
Peak serum levels of ALP, AST, and ALT did not differ significantly among the three groups with different magnitudes of renal infarction. However, peak serum levels of LDH, BUN, and creatinine were significantly higher in patients with severe renal infarction (p = 0.004, p = 0.015, and p < 0.001, respectively). The frequency of microscopic hematuria was not different among the three groups, whereas degree of proteinuria was positively correlated with severe infarction (p = 0.016) ().
Table 3. Comparison of laboratory findings according to magnitude of renal infarction.
Treatments and clinical course
After the initial use of unfractionated heparin or low-molecular-weight heparin in 72 patients, warfarin was administered in 66 patients, and the other six patients received aspirin. Only heparin was injected in another four patients, three of whom died during hospitalization. Four patients received only warfarin. The remaining 20 patients were treated conservatively owing to aortic dissection (n = 5), renal artery dissection after percutaneous transluminal angioplasty (n = 1), post-trauma (n = 5), bleeding (n = 5), and no adequate reason (n = 4).
Effects on renal function
AKI by renal infarction occurred in 30 patients during hospitalization. Renal infarct size was positively correlated with the occurrence of AKI (p = 0.004). The clinical symptoms and signs were not different between the AKI and non-AKI patients (data not shown). The AKI patients were significantly older than the non-AKI patients (p = 0.020), and peak serum AST, ALT, and LDH levels were significantly higher in patients with AKI (p < 0.001, p < 0.001, and p = 0.027, respectively). There was no difference in peak serum ALP between the two groups (p = 0.055). On dipstick test, microscopic hematuria were more frequent in the AKI group (p = 0.038) and degree of color reaction in part of protein was significantly correlated with occurrence of AKI (p < 0.001). White blood cell (WBC) counts were higher in the AKI group (p = 0.035) ().
Table 4. Comparison of clinical and laboratory parameters in the patients with renal infarction according to the development of AKI.
The managements of in patients with AKI mainly were mainly supportive such as fluid restriction and correction of fluid overload by furosemide, severe acidosis with bicarbonate infusion, and hyperkalemia. There was no one case of undergoing renal replacement therapy. Most of the patients recovered from AKI, except for seven patients who developed persistent renal impairment (CKD progression). In the AKI group, six patients died because of combined comorbidities, whereas no patients died in the non-AKI group (p < 0.001) (). There were no deaths due to renal infarction itself; three patients due to cerebral infarction, one patient due to superior mesentery artery infarction, one patient due to unknown etiology.
Table 5. Comparison clinical data in the patients with renal infarction according to with/without CRF.
Thirty-one patients, defined as CRF patients, had a baseline eGFR <60 mL/min. AKI was more common in patients with CRF, compared with non-CRF patients, whose baseline eGFR was >60 mL/min (p = 0.003). AKI already occurred in seven patients among CRF group at admission whereas in one patient among non-CRF group. AKI also newly occurred in 10 patients among CRF group during hospitalization whereas in 12 patients among non-CRF group (p = 0.045). However, there was no difference in the rate of CKD progression and death between CRF patients and non-CRF patients (4 vs. 3 patients, p = 0.21, 3 vs. 3 patients, p = 0.1, respectively).
CKD progression after renal infarction occurred in seven patients (23.3%). Renal infarct size was positively correlated with the progression of CKD (p = 0.015), with 57% of the patients in the CKD progression group having >50% renal infarct magnitude, compared with 13% of the patients in the non-CKD progression group (). The clinical symptoms and signs were not different between the CKD progression and non-CKD progression groups (data not shown), and age did not different between the two groups (p = 0.371). Peak serum ALP was higher in the CKD progression group (p = 0.028), but peak serum AST, ALT, and LDH levels were not significantly different between the two groups (p = 0.863, p = 0.710, and p = 0.214, respectively). There was no difference in the frequency of microscopic hematuria (p = 1.000) or proteinuria (p = 0.104) between the two groups.
Table 6. Comparison of clinical and laboratory parameters in the patients with renal infarction according to the progression of CKD.
Discussion
The results of this study demonstrated that AKI commonly occurred after acute renal infarction and was positively associated with the magnitude of renal infarction. AKI was usually accompanied by high serum AST, ALT, and LDH levels; a high WBC count; and high frequencies of hematuria and higher degree of proteinuria. AKI by renal infarction more frequently occurred in patients with CRF. Renal impairment by acute renal infarction generally improved with conservative or therapeutic management, although some patients progressed to chronic renal disease. Furthermore, this CKD progression was also correlated with the magnitude of renal infarction.
Acute renal impairment by acute renal infarction has been rarely reported. The reported incidence of AKI ranges from 0% to 60% in study populations of different sizes.Citation3,Citation9–11 The cumulative incidence was 44% in a study that included data of all renal infarction patients found in the medical literature.Citation12 However, these studies only described the characteristics of renal infarction and did not focus on renal function as affected by renal infarction. In addition, because they did not consider baseline renal function, the definitions of acute renal impairment and chronic renal disease were ambiguous. These studies did not describe renal impairment prognosis affected by renal infarction or the risk factors for AKI in these patients. In the present study, a focus on how renal function is affected by acute renal infarction helped to clarify the definitions of AKI and CKD, to identify factors associated with renal impairment, and to evaluate the prognosis of renal function after renal infarction.
In the present study, acute renal infarction occurred in 31 CRF patients with an eGFR <60 mL/min, and 4 of these patients did not recover renal function. In contrast, 3 of 69 patients with an eGFR >60 mL/min had CKD progression. Furthermore, AKI already occurred in seven patients among CRF group at admission whereas in one patient among non-CRF group. These findings suggest that residual renal function is important for the prevention of AKI by acute renal infarction in the early stages, but there is no difference in long-term recovery capacity. The average eGFR in CRF patients was 46 mL/min. This residual kidney capacity should be adequate for renal function recovery in the long run, although these patients are vulnerable to acute injury by acute renal infarction.
Elevated serum LDH is a highly sensitive marker for cell necrosis in patients with acute renal infarction.Citation3,Citation9–11,Citation13 LDH was elevated in nearly all (96.4%) of the patients in this study during hospitalization and was significantly associated with the magnitude of renal infarction. LDH was also significantly elevated in patients with AKI, suggesting that a greater magnitude of renal infarction induces a higher incidence of renal impairment.
Hematuria and proteinuria during renal infarction might be caused by glomerular and tubular damage resulting from tissue necrosis. Microscopic hematuria was shown in 60% of the patients whereas proteinuria is 56% of the patients in the present study. This frequency might be of an underestimation because hematuria and proteinuria caused by tissue necrosis can be considerably delayed and urinalysis was usually performed only once upon admission in the present patients, with no follow-up urinalysis. Proteinuria was positively correlated with the magnitude of renal infarction and the occurrence of AKI, providing further evidence that AKI might be associated with the magnitude of renal involvement in renal infarction. However, microscopic hematuria was not significantly associated with the magnitude of renal infarction, which we cannot explain at this time although microscopic hematuria was positively correlated with the occurrence of AKI. These results suggest that renal proteinuria might be a better predictor of AKI and renal prognosis in patients with acute renal infarction.
Our study has several limitations. A retrospective analysis is not ideal because laboratory data can be incomplete due to patient referral and incorrect initial or maximal laboratory values. The etiology of renal infarction may also be incomplete, although we did our best to categorize and evaluate the etiology in our patients. Furthermore, the baseline creatinine values might have been incorrect in some cases because we sometimes used baseline creatinine values from other hospitals. Nevertheless, our data reliably demonstrate that serum creatinine values did not differ greatly among hospitals. Finally, the etiology of AKI may not necessarily involve acute renal infarction; all of the patients underwent enhanced CT for diagnosis, and the contrast dye can induce AKI. In our study, we clinically could not distinguish radiocontrast-induced AKI from it by renal infarction. Radiocontrast-induced AKI has a lower incidence in populations with no underlying renal impairment.Citation14,Citation15 As the average eGFR in 30 patients with AKI in this study was 75 mL/min and the average eGFR in CRF patients was 46 mL/min, the frequency of radiocontrast-induced AKI was suspected to be likely low. This suggests that AKI occurrence in our group might be due to renal infarction itself. Despite these limitations, our study results are meaningful due to the large number of enrolled patients and the focus on changes in renal function associated with renal infarction.
In conclusion, AKI frequently occurred in patients with acute renal infarction, especially in patients with underlying renal impairment. Although most AKI cases recover spontaneously, renal impairment following acute renal infarction can persist. Thus, early diagnosis and intervention are needed to preserve renal function.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lessman RK, Johnson SF, Coburn JW, et al. Renal artery embolism: Clinical features and long-term follow-up of 17 cases. Ann Intern Med. 1978;89:477–482
- Michael Y, Francisco L. Disorders of the renal arteries and veins. In: Brenner BM. Brenner & Rector's the Kidney. 7th ed. Philadelphia: W. B. Saunders Company; 2008:1162–1169
- Domanovits H, Paulis M, Nikfardjam M, et al. Acute renal infarction: Clinical characteristics of 17 patients. Medicine. 1999;78:386–394
- Braun DR, Sawczuk IS, Axelrod SA. Idiopathic renal infarction. Urology. 1995;45:142–145
- Lumerman JH, Hom D, Eiley D, et al. Heightened suspicion and rapid evaluation with CT for early diagnosis of partial renal infarction. J Endourol. 1999;13:209–214
- Suzer O, Shirkhoda A, Jafri SZ, et al. CT features of renal infarction. Eur J Radiol. 2002;44:59–64
- Mehta R, Kellum J, Shah S, et al. AKI network: Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11:R31
- Stevens LA, Coresh J, Greene T, et al. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483
- Korzets Z, Plotkin E, Bernheim J, et al. The clinical spectrum of acute renal infarction. Isr Med Assoc J. 2002;4:781–784
- Hazanov N, Somin M, Attali M, et al. Acute renal embolism. Forty-four cases of renal infarction in patients with atrial fibrillation. Medicine (Baltimore). 2004;83:292–299
- Huang CC, Lo HC, Huang HH, et al. ED presentations of acute renal infarction. Am J Emerg Med. 2007;25:164–169
- Antopolsky M, Simanovsky N, Stalnkowicz R, et al. Renal infarction in the ED: 10-year experience and review of the literature. Am J Emerg Med. 2012;30:1055–1060
- Huang CC, Chen WL, Chen JH, et al. Clinical characteristics of renal infarction in an Asian population. Ann Acad Med Singapore. 2008;37:416–420
- Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;16:1416–1420
- Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;16:180–184

