Abstract
The role of uric acid (UA) on the pathogenesis and progression of chronic kidney disease (CKD) remains controversial. Experimental and clinical studies indicate that UA is associated with several risk factors of CKD including diabetes, hypertension, oxidative stress, and inflammation and hyperuricemia could be considered as a common dominator linking CKD and cardiovascular disease. Notably, the impact of serum UA levels on the survival of CKD, dialysis patients, and renal transplant recipients is also a matter of debate, as there are conflicting results from clinical studies. At present, there is no definite data whether UA is causal, compensatory, coincidental or it is only an epiphenomenon in these patients. In this article, we attempt to review and elucidate the dark side of this old molecule in CKD and renal transplantation.
Introduction
Uric acid (UA) is the end product of purine nucleotides metabolic breakdown. Xanthine oxidase (XO), which is a form of xanthine oxidoreductase, catalyzes the final oxidation of hypoxanthine and xanthine to UA.Citation1
The kidneys eliminate approximately 70% of the UA load while 30% is eliminated by the gastrointestinal tract.Citation1 UA is filtered by the glomeruli and the amount of UA excretion depends on its reabsorption and secretion mainly at the proximal tubule. Above 5–10% of the filtered UA is excreted in the urine, while 90% is reabsorbed.Citation1
Hyperuricemia (HUA) is the result of the balance between UA production and excretion. In more than 90% of the cases, HUA is due to impaired renal excretion of UA.Citation1 The prevalence of HUA is increased as the glomerular filtration rate (GFR) declines, ranging from 40% to 70% in chronic kidney disease (CKD) stages 1–5.Citation2,Citation3 Decreased UA filtration due to reduced GFR, impaired UA tubular secretion caused by tubulointerstitial disease, and use of diuretics and cyclosporine (CsA) contribute to increased prevalence of HUA in CKD patients.Citation4 In terms of ethnic and gender differences, Asian patients with end-stage renal disease (ESRD) present higher rates of HUACitation5, and men have a higher risk of HUA and gout in the general population.Citation6
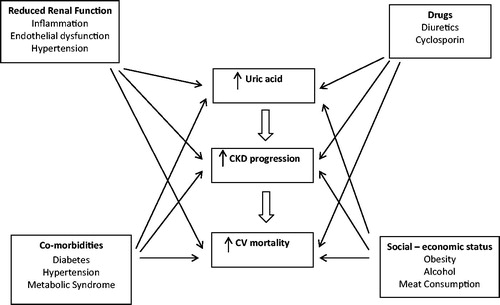
Data from several studies have associated HUA with increased incidence and/or progression of CKD. Diabetes is one of the leading causes of CKD and HUA has been associated with the pathogenesis of the microvascular complications of diabetes.Citation7 Findings from animal studies revealed a causal role of persistent HUA in the pathogenesis and development of hypertensionCitation8–10 which, in turn, is implicated in the progression of CKD and cardiovascular (CV) mortality.
UA has been associated with oxidative stress, inflammation, and endothelial dysfunctionCitation11 (). The impact of UA in all-cause and CV mortality of dialysis patients' remains controversial.Citation12,Citation13 Controversial also remains the role of UA in renal transplantation.Citation7
Table 1. Risk factors for CKD progression associated with hyperuricemia.
Relationship of HUA – chronic kidney disease incidence and progression
Several studies with conflicting results in some instance have investigated the relationship between HUA and CKD. Some studies reported an independent positive association of HUA with incident CKD,Citation14–19 progression of CKD, and ESRD.Citation2,Citation20–27 Interestingly, a positive association of HUA, IgA nephropathy, and lupus nephritis has also been reported.Citation28,Citation29 The majority of all these studies has been recently described elsewhere.Citation30,Citation31
On the contrary, this positive association between HUA and CKD progression has not been supported by other studies. A cohort study including 840 participants with CKD stages 3 and 4 reported that HUA was not an independent risk factor for CKD progression, but was correlated with increased risk for all-cause mortality and CV mortality.Citation3 In accordance, a prospective 7 years follow-up study including 227 Caucasian patients with non-diabetic CKD, revealed that HUA did not predict CKD progression.Citation32
These contradictory results may be explained by the differences in the case mix and the follow-up period as well as gender differences (higher incidence of HUA in men), and different thresholds of HUA in these studies. Thus, some authorities support that UA is not a real risk factor for CKD. In addition, given the decreased clearance of UA, even in the early stages of CKD, HUA might be seen as an epiphenomenon and not a real cause of CKD. In this respect, HUA may just reflect the severity of CKD (advanced stage).
Data from animal models with HUA caused by the administration of low doses of oxonic acid (uricase inhibitor), suggested a causal role for UA in the progression of CKD.Citation8 A renal vascular disease characterized by renal cortical vasoconstriction, afferent arteriolopathy, and glomerular hypertension has been observed in this animal model.Citation8,Citation9,Citation33 In addition, light microscopy revealed tubular dilatation, renal cortex atrophy, and to some extent glomerulosclerosis.Citation33 Notably, activation of renin–angiotensin system (RAS), enhanced oxidative stress, alterations in endothelin-1 expression, and increased expression of cyclooxygenase-2 (COX2) have been proposed to explain endothelial and vascular abnormalities observed in animals with HUA.Citation7 In support, administration of febuxostat which is a XO inhibitor alleviated systemic and glomerular hypertension and blockage of RAS and prevented pre-glomerular vasculopathy in animal models with HUA.Citation34,Citation35 Interestingly, a higher grade of proteinuria, increased blood pressure, and higher serum creatinine levels have been observed in rats with CKD (remnant kidney model) and HUA compared with remnant kidney rats without HUA.Citation36 Moreover, renal hypertrophy, glomerulosclerosis, and interstitial fibrosis were more pronounced in hyperuricemic rats.Citation36
Notably, findings from animal models are usually specie specific and may provide some evidence, but do not necessarily translate into the human underlying disease process. So, extrapolation of experimental animal findings to human disease is not always straightforward.
An interesting approach to investigate the role of UA on CKD progression is to evaluate the effect of decreasing UA levels on CKD. Several studies reported a beneficial effect of UA lowering on CKD progression and UA has been proposed as a promising target of therapy in CKD patients.Citation37–43 Recently, Omori et al.Citation37 reported that in animal models with obstructive nephropathy, febuxostat inhibited renal interstitial inflammation and fibrosis suggesting a therapeutic value in slowing CKD progression. In accordance, Sakai et al.Citation42 reported that febuxostat by lowering UA levels in CKD patients may suppress the progression of CKD. Notably, data from a recent meta-analysis supported that allopurinol may slow the progression of CKD.Citation43 However, in another meta-analysis including eight trials, an uncertain effect of allopurinol has been reported.Citation44
Finally, the relative importance of all these associations is yet controversial. One difficulty is to determine whether UA per se is a risk factor for the development and progression of CKD. Given the high incidence of UHA due to decreased clearance of UA in CKD, it could be assumed that the increased levels of UA in CKD present only as an epiphenomenon. In this respect, CKD could be considered the cause of HUA and not the reverse. Moreover, the independence of a risk factor is not necessarily equal to causality and a risk factor may be independent and not causal, when the real causal parameter is not included in the statistical analysis.Citation45 In the same way, a risk factor may be not independent but it could still be causal.Citation45 It could be assumed that HUA is an indirect cause of kidney disease, but the difficulty to clarify the role of UA as an independent risk factor for CKD is mainly due to the complex correlations and interactions between UA and conventional risk factors for CKD. However, the elucidation of the relationship between UA and CKD progression remains an evolving area of research.
Gouty nephropathy and CKD
Findings from early studies in rats provided evidence that HUA in combination with hyperuricosuria may lead to kidney disease through intraluminal deposition of crystals in the collecting duct of the nephron in a manner simulating gouty arthropathy.Citation46–48 UA crystals adhere to the surface of renal epithelial cells and may induce an acute local inflammatory response.Citation49 Interestingly, UA induces phenotypic transition of renal tubular cells suggesting a novel potential mechanism of CKD.Citation50 Apart from the increased risk of developing kidney stones, such effects seem to contribute to a decrease in GFR aggravated by the presence of hypertension.Citation51 In the early 1960s, Talbott and Terplan,Citation52 reported that 10–20% of patients with untreated gout developed ESRD. Moreover, renal biopsies in these patients revealed tubulointerstitial fibrosis, arteriosclerosis, and glomerulosclerosis.Citation52 In subsequent years, the causal role of HUA and gout in kidney disease onset and progression had been the subject of a large debate. Some investigators suggested that the histological findings revealed in renal biopsy specimens in patients with HUA such as tubulointerstitial fibrosis, arteriosclerosis, and glomerulosclerosis were similar with those observed in hypertensive renal disease.Citation45 These non-specific alterations were also present in advanced age or in patients with asymptomatic HUA. Moreover, given that patients with HUA are usually hypertensive, it is difficult to attribute such non-specific lesions to UA or to hypertension. Notably, Nickeleit and MihatschCitation53 concluded that renal tophi hardly lead to terminal irreversible renal failure based on autopsy cases with gout. Additionally, gout is correlated with several other risk factors including diabetes, advanced age, and male sex. Thus, it is difficult to dissect the causal effect of UA from the synergic effect of the other risk factors. Therefore, the term gouty nephropathy has been considered as a misnomer by some authorities and, in the past, it was removed from the list of causes of CKD in the classical nephrology textbooks.
HUA, diabetes, and diabetic nephropathy
Diabetic nephropathy is one of the leading causes of ESRD. HUA promotes insulin resistance and concomitant hyperinsulinemia.Citation54,Citation55 Zhang et al.Citation56 reported that HUA contributes to abnormal glucose metabolism by increasing oxidative stress and by inhibiting the function of pancreatic β cells. Kidney tubular function seems to be influenced by insulin metabolic signaling and renal UA clearance decreases with reducing insulin-mediated glucose disposal.Citation57 Obesity predisposes to type 2 diabetes and in obese patients, especially in those with increase high-fructose diet, an increase hepatic production of UA has been reported, suggesting a potential link among obesity, diabetes, and UA.Citation58
Data from animal studies are limited. Kosugi et al.Citation59 reported that treatment of diabetic mice with a allopurinol for 8 weeks, reduced albuminuria, and ameliorated tubulointerstitial injury. The authors concluded that the protective mechanism was the reduction of local inflammation. Kim et al.Citation60 found that lowering UA with allopurinol retarded diabetic nephropathy in animal models with type 2 diabetes, through attenuation of the transforming growth factor-β1 and Smad pathway-induced profibrogenic tubular changes.
Data from clinical studies supported the concept that HUA is a risk factor for diabetic nephropathy. Hovind et al.Citation61 reported that baseline UA levels predicted persistent macro-albuminuria in patients with type 1 diabetes. In accordance, findings from the Coronary Artery Calcification in Type 1 Diabetes Study supported that high serum UA levels predict the development of albuminuria.Citation62 Moreover, the authors reported that every 1 mg/dL increase in serum UA levels may lead to an 80% increased risk for micro- or macroalbuminuria.Citation62 Ficociello et al.Citation63 have found an association between serum UA levels and early GFR loss in 355 participants with type 1 diabetes. In an observational, cross sectional study, high UA serum levels were associated with a decline in GFR in type 1 diabetic patients with normo- or microalbuminuria.Citation64 Similar, serum UA levels were correlated with micro and macro-vascular complications in type 2 diabetic patients.Citation65 Data from a another study, including 1449 type 2 diabetic patients with preserved kidney function and a 5 years follow-up period, reported that the presence of HUA doubled the risk of incident CKD. The authors concluded that HUA is an independent risk factor for the development of CKD in type 2 diabetic patient.Citation66 Additionally, Kim et al.Citation67 reported that increased levels of UA may predict the occurrence of advanced CKD in 512 type 2 diabetics with preserved renal function. Interestingly, Bo et al.Citation68 reported that HUA in type 2 diabetes is correlated with early onset or increased progression of diabetic nephropathy, whereas the opposite occurs with hypouricemia. In another study from Japan including 1213 type 2 diabetic patients, a positive independent association among HUA, coronary heart disease, and renal dysfunction was reported, supporting the hypothesis that HUA is implicated in the pathogenesis of the micro- and macro-vascular diabetic complications.Citation69 In accordance, Tanaka et al.Citation70 reported that HUA was correlated with increased risk of macrovascular disease in diabetic CKD patients.
The impact of lowering UA on the progression of diabetic nephropathy is challenging, but few studies have been conducted in this field. Momeni et al.Citation71 investigated the effect of allopurinol administration in 40 patients with type 2 diabetic nephropathy. The authors reported a decrease in proteinuria after 4 months of treatment.Citation71 However, the small size, the short follow-up period, and the lack of assessment of renal function do not permit safe conclusions. According to the Reduction in Endpoints with the Angiotensin Antagonist Losartan (RENAAL) study, an angiotensin II antagonist (Losartan), apart its effect on lowering blood pressure and proteinuria, has also an UA lowering effect suggesting that the renoprotective effect of losartan may be partially attributed to decreased serum UA levels.Citation72 Moreover, the renoprotective effect of losartan was decreased from 22% to 17% after adjusting for the effect of allopurinol on serum UA levels, suggesting that UA may partially contribute to diabetic nephropathy progression.Citation72
HUA, hypertension, and CKD
Several studies reported that HUA predicts a higher risk of hypertension, independent of other risk factors.Citation73–83 Interestingly, HUA is associated even with prehypertension, especially when microalbuminuria is present.Citation84,Citation85 In the majority of these studies, HUA precedes the onset of hypertension indicating that it is not a result of hypertension. However, Forman et al.Citation86 reported that HUA was not a risk factor for incident hypertension among 750 participants who developed hypertension after the age of 60 years. It is worth noting that HUA is not usually associated with secondary hypertension, impoverishing the hypothesis that HUA is an epiphenomenon of hypertension.Citation87 Interestingly, HUA is not only associated with hypertension but also with its complications including left ventricular hypertrophy.Citation88,Citation89 However, Tsioufis et al.Citation90 did not confirm this finding.
Findings from several studies provided a direct evidence that UA is implicated in the pathogenesis of hypertension.Citation8--10,Citation91 Additionally, the microvascular changes caused by UA remain after correction of blood pressure indicating that UA contributes to microvascular disease independently of hypertension.Citation92 Interestingly, data from in vitro studies reported that UA induces proliferation of vascular smooth-muscle cells, local inflammation, oxidative stress, endothelial dysfunction, and local RAS activation.Citation93
Recently, Sheikhbahaei et al.Citation94 reported a synergistic effect between UA and various components of metabolic syndrome including hypertension and diabetes, on CKD odds. In this respect, it could be assumed that UA contributes to CKD development and progression through conventional CKD risk factors such as diabetes and hypertension, indicating an indirect effect. In support, data from a recent prospective cohort study involving 2601 individuals reported that increased serum UA levels were independently associated with a faster decline of GFR and incident CKD and this association was stronger in hypertensive individuals. The authors concluded that hypertension mediates the correlation between high serum UA levels and CKD.Citation95
Data from experimental and clinical studies reported that lowering UA by using XO inhibitors may lead to a decrease in blood pressure, supporting a direct effect of UA.Citation8,Citation96–98 However, Gois and SouzaCitation99 reported that there is insufficient evidence of an antihypertensive effect of allopurinol.
Finally, based on the experimental data, there is evidence of a direct action of UA on hypertension-induced kidney injury through RAS activation, inflammation, endothelial dysfunction, and oxidative stress. Thus, the majority of patients with hypertension and HUA should present CKD, but actually this does not occur. Probably, UA in some instance mediates and contributes to CKD development and progression through hypertension-induced renal injury suggesting an indirect action on the pathogenesis of kidney disease. Another hypothesis is that CKD results in HUA, which, in turn, through hypertension-induced changes and endothelial dysfunction, aggravates the decline in renal function. Nevertheless, all these theories remain to be proven.
HUA, oxidative stress, and inflammation
Oxidative stress is implicated both in CKD and in CV disease (CVD) progression and it is characterized by increased production of free radicals and reactive oxygen species (ROS).Citation100 On one hand, in humans, XO does not permit further oxidation of UA.Citation100 During this process, ROS are generated which play a crucial role in the enhanced vascular oxidative stress observed in both CKD and CVD. Based on the clinical data that HUA may contribute to CKD and CVD progression, some therapeutic strategies are oriented on the inhibition of the increased XO activity. It could be assumed that the potential benefit of allopurinol is not due to its UA lowering effect, but to an antioxidant action attributed to the decreased XO activity.Citation11 Moreover, findings from animal studies supported that UA itself may promote endothelial dysfunction by inhibition of nitric oxide function.Citation11
On the other hand, UA may have a protective role under certain conditions. Nieto et al.Citation101 reported that UA plays a protective role as an antioxidant in atherosclerosis and this is a probable compensatory mechanism in order to counteract oxidative damage attributed to atherosclerosis. In accordance, both in vitro and in vivo studies support that UA is an important antioxidant by acting as a free radical scavenger and a potent chelator of transitional metal ions.Citation102,Citation103 Kurra et al.Citation104 reported that HUA reduced oxidative stress in animal models with renal insufficiency. However, the real determinative factors of UA action on oxidative process have not been elucidated. Lippi et al.Citation105 in an attempt to explain the paradox action of UA reported that in certain situations, the antioxidant compounds may become pro-oxidant, especially when they are at supranormal levels in blood. Thus, it could be postulated that UA concentration determines its double controversial action. Urate at low concentrations may have a beneficial action as an antioxidant; while at higher concentrations, it may promote oxidative stress reflecting a rather maladaptive/compensatory mechanism in order to slow the progression of kidney damage. However, a threshold has not been yet specified. Another hypothesis is that UA may have different biochemical effects under different pathological or physiological conditions and in different cellular environments. Recently, Johnson et al.Citation31 reported that on one hand UA is a pro-oxidant in the intracellular environment by stimulating NADPH oxidases, while outside the cell is a potent antioxidant. On the other hand, it could be hypothesized that XO activity and not UA itself plays a crucial role on oxidative process but all these hypothesis remain to be proven.
Both CKD and CVD are characterized by low-grade inflammation. Inflammation may be considered as a potential underlying mechanism linking HUA, CKD, and CVD. In vascular smooth muscle cells, UA promotes inflammation by activating NF-κB and by enhancing serum C-reactive protein (CRP) expression.Citation106,Citation107 Moreover, HUA was associated with several circulating inflammatory cytokines.Citation108 Interestingly, C reactive protein (CRP) in combination with HUA may interact on the development of albuminuria and concomitant CKD progression on type 2 diabetic patients.Citation109 A significant positive correlation between CRP and serum UA levels was observed in 337 CKD patients.Citation110 In this regard, it could be assumed that HUA is a marker of inflammation associated with both CKD and CVD.
Several studies provided evidence that UA mediates endothelial dysfunction contributing to CKD and CVD progression. Endothelial dysfunction with HUA in the early stages of CKD is associated with micro-inflammation and oxidative stress.Citation111 More attention has been given to the xanthine oxidoreductase system since allopurinol may prevent the progression of atherosclerosis by reducing endothelial dysfunction.Citation112,Citation113 However, data from other studies did not support a direct effect of UA on endothelial dysfunction.Citation114
HUA and CV mortality
According to the Hemodialysis (HEMO) study, CVD is the leading cause of death in dialysis patients.Citation115 Moreover, there is a reciprocal relationship between CVD and CKD, and UA might be considered as a common dominator linking these two clinical conditions.
Similar to the general population, the evidence for a strong correlation between UA and CVD mortality in CKD patients is conflicting. Data from epidemiological studies in CKD patients revealed a direct linear or “J-shaped” correlation with all cause or CV mortality.Citation3,Citation116,Citation117 However, according to other studies, this correlation diminished after adjustment for GFR or proteinuria.Citation118,Citation119 Moreover, Madero et al.Citation3 found that CKD patients with high serum UA concentration had a higher risk for all-cause and CV mortality. Similar, Kanbay et al.Citation120 reported that HUA predicts CV events in advanced CKD stages. In accordance, Liu et al.Citation116 suggested that HUA is a risk factor for all-cause and CV events but not for renal outcomes in stages 3–5 CKD patients. Additionally, HUA independently correlates with CV mortality and this correlation is stronger with decreasing GFR.Citation121 Interestingly, Chung et al.Citation122 revealed that HUA is an independent risk factor for all-cause mortality in CKD patients undergoing percutaneous coronary intervention but not in those without CKD. Notably, allopurinol administration in hypertensive CKD patients prevents CV and all-cause mortality.Citation123
In the hemodialysis (HD) population, the data are more contradictory. Hsu et al.Citation12 have found a “J-shaped” association between UA and all-cause mortality in 146 HD patients. Recently, Lee et al.Citation124 reported that serum UA levels were independently associated with valvular and vascular calcifications in 81 HD patients. Additionally, Antunovic et al.Citation125 reported that HUA may predict all-cause and CV mortality in HD patients. In support, Lobo et al.Citation126 reported a positive association of UA levels and several markers of inflammation suggesting a potential role of HUA in the pathogenesis of atherosclerosis in HD patients. In addition, Cohen et al.Citation127 in a cohort of 259,209 patients from the United States Renal Data System evaluated the incidence of gout and its relationship with mortality. The authors calculated that an episode of gout was independently correlated with a 1.5-fold increase in mortality risk. The authors proposed inflammation and atherosclerosis as the underlying mechanisms of the increased mortality.Citation127
On the contrary, data from a retrospective study reported that low serum UA concentration in HD patients with hypoalbuminemia is a mortality risk factor.Citation128 Another large retrospective study involving 5827 HD patients reported that higher serum UA levels were correlated with lower risk of all-cause and CVD mortality.Citation13 These results might be explained by the reverse epidemiology. Interestingly, from ancient times, it was well known that gout was a disease of the Emperors of the Byzantium suggesting their better social-economic status compared with malnourished people.Citation129 In this respect, HUA may reflect a better nutritional status in HD patients and low UA levels may represent a form of protein energy wasting syndrome.
Contradictory results have been reported also in peritoneal dialysis (PD) patients. Data from two studies revealed an association of HUA with all-cause and CVD mortality.Citation130,Citation131 However, in a recent observational study including 2264 PD patients, only a weak correlation of HUA with all-cause mortality could be detected.Citation132 The lack of large prospective studies in this field does not lead to reliable conclusions.
HUA and renal transplantation
HUA occurs at the early post-transplant period. The use of diuretics and calcineurin inhibitors, reduced GFR, advanced recipient age, obesity, and pre-existing history of HUA are common risk factors.Citation133 CsA is the most common risk factor and conversion of CsA to tacrolimus or everolimus immunosuppression may decrease serum UA levels.Citation134,Citation135
The impact of HUA on graft survival is controversial. Some studies indicate that HUA is only a marker of graft dysfunction,Citation136–139 whereas some other studies suggest that UA is an independent risk factor for graft dysfunction.Citation140–146 Recently, Hart et al.Citation147 reported that HUA correlated with tubular atrophy and interstitial fibrosis in chronic allograft nephropathy. Similar to the experimental findings, HUA in humans may exacerbate CsA nephrotoxicity.Citation148 Additionally, CsA-induced hypertension and endothelial dysfunction may contribute to graft dysfunction.Citation149 Interestingly, an association among HUA, inflammation, graft dysfunction, and CV events has been reported.Citation142
While post-transplant gout attacks should be treated, the treatment of asymptomatic HUA remains controversial.Citation133 Minimization of diuretics and CsA and avoidance of purine-rich foods and alcohol are effective strategies to decrease UA concentrations. Considering the link of UA with diabetes and metabolic syndrome, hypertension and CVD risk, minimizing these risk factors may be proven beneficial for graft function.
Conclusion
After so many years of scientific research, the real significance of this old molecule is widely debated and it remains obscure whether UA is causal, compensatory, coincidental, or it is only an epiphenomenon of CKD. In accordance, nebulous also remains the relationship of UA with CV mortality. As HUA is usually associated with a better nutritional status, whereas hypo-uricemia with malnutrition, it could be speculated that the alterations of UA metabolism in advanced CKD and its reported associations may be just a reflection of different population characteristics, comorbidities, or social-economic status (). More prospective randomized and well-designed trials with a large number of participants are needed to clarify the exact relationship among UA, CKD, and CV mortality.
Declaration of interest
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008;75(Suppl. 5):S13–S16
- Chonchol M, Shlipak MG, Katz R, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50(2):239–247
- Madero M, Sarnak MJ, Wang X, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53(5):796–803
- Murea M. Advanced kidney failure and hyperuricemia. Adv Chronic Kidney Dis. 2012;19(6):419–424
- Ohno I, Yamaguchi Y, Saikawa H, et al. Sevelamer decreases serum uric acid concentration through adsorption of uric acid in maintenance hemodialysis patients. Intern Med. 2009;48(6):415–420
- Singh JA. Racial and gender disparities among patients with gout. Curr Rheumatol Rep. 2013;15(2):307
- Bellomo G. Uric acid and chronic kidney disease: A time to act? World J Nephrol. 2013;2(2):17–25
- Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106
- Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282(6):F991–F997
- Watanabe S, Kang DH, Feng L, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360
- Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25(1):39–42
- Hsu SP, Pai MF, Peng YS, Chiang CK, Ho TI, Hung KY. Serum uric acid levels show a ‘J-shaped’ association with all-cause mortality in hemodialysis patients. Nephrol Dial Transplant. 2004;19(2):457–462
- Latif W, Karaboyas A, Tong L, et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol. 2011;6(10):2470–2477
- Domrongkitchaiporn S, Sritara P, Kitiyakara C, et al. Risk factors for development of decreased kidney function in a southeast Asian population: A 12-year cohort study. J Am Soc Nephrol. 2005;16(3):791–799
- Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19(6):1204–1211
- Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19(12):2407–2413
- Sonoda H, Takase H, Dohi Y, Kimura G. Uric acid levels predict future development of chronic kidney disease. Am J Nephrol. 2011;33(4):352–357
- Wang S, Shu Z, Tao Q, Yu C, Zhan S, Li L. Uric acid and incident chronic kidney disease in a large health check-up population in Taiwan. Nephrology (Carlton). 2011;16(8):767–776
- Mok Y, Lee SJ, Kim MS, Cui W, Moon YM, Jee SH. Serum uric acid and chronic kidney disease: The Severance cohort study. Nephrol Dial Transplant. 2012;27(5):1831–1835
- Kuo CF, Luo SF, See LC, et al. Hyperuricaemia and accelerated reduction in renal function. Scand J Rheumatol. 2011;40(2):116–121
- Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–350
- Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642–650
- Iseki K, Iseki C, Kinjo K. Changes in serum uric acid have a reciprocal effect on eGFR change: A 10-year follow-up study of community-based screening in Okinawa, Japan. Hypertens Res. 2013;36(7):650–654
- Bellomo G, Venanzi S, Verdura C, Saronio P, Esposito A, Timio M. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010;56(2):264–272
- Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10(6):403--409
- Yen CJ, Chiang CK, Ho LC, et al. Hyperuricemia associated with rapid renal function decline in elderly Taiwanese subjects. J Formos Med Assoc. 2009;108(12):921–928
- Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17(5):1444–1452
- Shi Y, Chen W, Jalal D, et al. Clinical outcome of hyperuricemia in IgA nephropathy: A retrospective cohort study and randomized controlled trial. Kidney Blood Press Res. 2012;35(3):153–160
- Yang Z, Liang Y, Xi W, Zhu Y, Li C, Zhong R. Association of serum uric acid with lupus nephritis in systemic lupus erythematosus. Rheumatol Int. 2011;31(6):743–748
- Jalal DI, Chonchol M, Chen W, Targher G. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61(1):134–146
- Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol Dial Transplant. 2013;28(9):2221–2228
- Sturm G, Kollerits B, Neyer U, Ritz E, Kronenberg F. MMKD Study Group. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008;43(4):347–352
- Sánchez-Lozada LG, Tapia E, Avila-Casado C, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol. 2002;283(5):F1105–F1110
- Sánchez-Lozada LG, Tapia E, Soto V, et al. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrol Dial Transplant. 2008;23(4):1179–1185
- Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26(2):269–275
- Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13(12):2888–2897
- Omori H, Kawada N, Inoue K, et al. Use of xanthine oxidase inhibitor febuxostat inhibits renal interstitial inflammation and fibrosis in unilateral ureteral obstructive nephropathy. Clin Exp Nephrol. 2012;16(4):549–556
- Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39(4):1227–1233
- Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59
- Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393
- Sezer S, Karakan S, Atesagaoglu B, Acar FN. Allopurinol reduces cardiovascular risks and improves renal function in pre-dialysis chronic kidney disease patients with hyperuricemia. Saudi J Kidney Dis Transpl. 2014;25(2):316–320
- Sakai Y, Otsuka T, Ohno D, Murasawa T, Sato N, Tsuruoka S. Febuxostat for treating allopurinol-resistant hyperuricemia in patients with chronic kidney disease. Ren Fail. 2014;36(2):225–231
- Wang H, Wei Y, Kong X, Xu D. Effects of urate-lowering therapy in hyperuricemia on slowing the progression of renal function: A meta-analysis. J Ren Nutr. 2013;23(5):389–396
- Bose B, Badve SV, Hiremath SS, et al. Effects of uric acid-lowering therapy on renal outcomes: A systematic review and meta-analysis. Nephrol Dial Transplant. 2014;29(2):406–413
- Mohandas R, Johnson RJ. Uric acid levels increase risk for new-onset kidney disease. J Am Soc Nephrol. 2008;19(12):2251–2253
- Gonick HC, Rubini ME, Gleason IO, Sommers SC. The renal lesion in gout. Ann Intern Med. 1965;62:667–674
- Bluestone R, Waisman J, Klinenberg JR. Chronic experimental hyperuricemic nephropathy. Lab Invest. 1975;33(3):273–279
- Waisman J, Mwasi LM, Bluestone R, Klinenberg JR. Acute hyperuricemic nephropathy in rats. An electron microscopic study. Am J Pathol. 1975;81(2):367–378
- Zhou Y, Fang L, Jiang L, et al. Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PLoS One. 2012;7(6):e39738
- Ryu ES, Kim MJ, Shin HS, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 2013;304(5):F471–F480
- Tarng DC, Lin HY, Shyong ML, Wang JS, Yang WC, Huang TP. Renal function in gout patients. Am J Nephrol. 1995;15(1):31–37
- Talbott JH, Terplan KL. The kidney in gout. Medicine (Baltimore). 1960;39:405–467
- Nickeleit V, Mihatsch MJ. Uric acid nephropathy and end-stage renal disease – Review of a non-disease. Nephrol Dial Transplant. 1997;12(9):1832–1838
- Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1(1):5–12
- Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61(5):943–947
- Zhang Y, Yamamoto T, Hisatome I, et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol. 2013;375(1-2):89–96
- Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–3011
- Khitan Z, Kim DH. Fructose: A key factor in the development of metabolic syndrome and hypertension. J Nutr Metab. 2013;2013:682673
- Kosugi T, Nakayama T, Heinig M, et al. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297(2):F481–F488
- Kim SM, Choi YW, Seok HY, et al. Reducing serum uric acid attenuates TGF-β1-induced profibrogenic progression in type 2 diabetic nephropathy. Nephron Exp Nephrol. 2012;121(3-4):e109–e121
- Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: An inception cohort study. Diabetes. 2009;58(7):1668–1671
- Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: Findings from the coronary artery calcification in type 1 diabetes study. Nephrol Dial Transplant. 2010;25(6):1865–1869
- Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: Results of a 6-year follow-up. Diabetes Care. 2010;33(6):1337–1343
- Rosolowsky ET, Ficociello LH, Maselli NJ, et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3(3):706–713
- Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in Thai patients with type 2 diabetes. J Diabetes Complications. 2014;28(2):124–129
- Zoppini G, Targher G, Chonchol M, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35(1):99–104
- Kim WJ, Kim SS, Bae MJ, et al. High-normal serum uric acid predicts the development of chronic kidney disease in patients with type 2 diabetes mellitus and preserved kidney function. J Diabetes Complications. 2014;28(2):130–134
- Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: Two different phenotypes. Eur J Clin Invest. 2001;31(4):318–321
- Ito H, Abe M, Mifune M, et al. Hyperuricemia is independently associated with coronary heart disease and renal dysfunction in patients with type 2 diabetes mellitus. PLoS One. 2011;6(11):e27817
- Tanaka K, Hara S, Kushiyama A, et al. Risk of macrovascular disease stratified by stage of chronic kidney disease in type 2 diabetic patients: Critical level of the estimated glomerular filtration rate and the significance of hyperuricemia. Clin Exp Nephrol. 2011;15(3):391–397
- Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4(2):128–132
- Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: A post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58(1):2–7
- Hunt SC, Stephenson SH, Hopkins PN, Williams RR. Predictors of an increased risk of future hypertension in Utah: A screening analysis. Hypertension. 1991;17(6Pt2):969–976
- Imazu M, Yamamoto H, Toyofuku M, et al. Hyperinsulinemia for the development of hypertension: Data from the Hawaii–Los Angeles–Hiroshima Study. Hypertens Res. 2001;24(5):531–536
- Alper AB Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: The Bogalusa Heart Study. Hypertension. 2005;45(1):34–38
- Jossa F, Farinaro E, Panico S, et al. Serum uric acid and hypertension: The Olivetti Heart Study. J Hum Hypertens. 1994;8(9):677–681
- Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49(2):298–303
- Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42(4):474–480
- Mellen PB, Bleyer AJ, Erlinger TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: The atherosclerosis risk in communities study. Hypertension. 2006;48(6):1037–1042
- Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27(11):835–841
- Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: The Normative Aging Study. Hypertension. 2006;48(6):1031–1036
- Sundström J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45(1):28–33
- Taniguchi Y, Hayashi T, Tsumura K, Endo G, Fujii S, Okada K. Serum uric acid and the risk for hypertension and type 2 diabetes in Japanese men: The Osaka Health Survey. J Hypertens. 2001;19(7):1209–1215
- Syamala S, Li J, Shankar A. Association between serum uric acid and prehypertension among US adults. J Hypertens. 2007;25(8):1583–1589
- Lee JE, Kim YG, Choi YH, Huh W, Kim DJ, Oh HY. Serum uric acid is associated with microalbuminuria in prehypertension. Hypertension. 2006;47(5):962–967
- Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. 2007;18(1):287–292
- Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–252
- Yoshimura A, Adachi H, Hirai Y, et al. Serum uric acid is associated with the left ventricular mass index in males of a general population. Int Heart J. 2014;55(1):65–70
- Nitta K, Iimuro S, Imai E, et al. Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(5):730–742
- Tsioufis C, Chatzis D, Vezali E, et al. The controversial role of serum uric acid in essential hypertension: Relationships with indices of target organ damage. J Hum Hypertens. 2005;19(3):211–217
- Boban M, Kocic G, Radenkovic S, et al. Circulating purine compounds, uric acid, and xanthine oxidase/dehydrogenase relationship in essential hypertension and end stage renal disease. Ren Fail. 2014;36(4):613--618
- Feig DI, Nakagawa T, Karumanchi SA, et al. Hypothesis: Uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int. 2004;66(1):281–287
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821
- Sheikhbahaei S, Fotouhi A, Hafezi-Nejad N, Nakhjavani M, Esteghamati A. Serum uric acid, the metabolic syndrome, and the risk of chronic kidney disease in patients with type 2 diabetes. Metab Syndr Relat Disord. 2014;12(2):102–109
- Sedaghat S, Hoorn EJ, van Rooij FJ, et al. Serum uric acid and chronic kidney disease: The role of hypertension. PLoS One. 2013;8(11):e76827
- Jalalzadeh M, Nurcheshmeh Z, Mohammadi R, Mousavinasab N, Ghadiani MH. The effect of allopurinol on lowering blood pressure in hemodialysis patients with hyperuricemia. J Res Med Sci. 2012;17(11):1039–1046
- Pai BH, Swarnalatha G, Ram R, Dakshinamurty KV. Allopurinol for prevention of progression of kidney disease with hyperuricemia. Indian J Nephrol. 2013;23(4):280–286
- Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial). Circ J. 2013;77(8):2043–2049
- Gois PH, Souza ER. Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst Rev. 2013;1:CD008652
- Pasalic D, Marinkovic N, Feher-Turkovic L. Uric acid as one of the important factors in multifactorial disorders – Facts and controversies. Biochem Med (Zagreb). 2012;22(1):63–75
- Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: A reaction to atherosclerosis? Atherosclerosis. 2000;148(1):131–139
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci USA. 1981;78(11):6858–6862
- Squadrito GL, Cueto R, Splenser AE, et al. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys. 2000;376(2):333–337
- Kurra V, Eräranta A, Jolma P, et al. Hyperuricemia, oxidative stress, and carotid artery tone in experimental renal insufficiency. Am J Hypertens. 2009;22(9):964–970
- Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta. 2008;392(1-2):1–7
- Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41(6):1287–1293
- Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562
- Lyngdoh T, Marques-Vidal P, Paccaud F, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011;6(5):e19901
- Ling Y, Li XM, Gao X. Cross-sectional association of serum C-reactive protein and uric acid with albuminuria in Chinese type 2 diabetic patients. Chin Med J (Engl). 2013;126(21):4023–4029
- Caravaca F, Martín MV, Barroso S, et al. Serum uric acid and C-reactive protein levels in patients with chronic kidney disease. Nefrologia. 2005;25(6):645–654
- Wang Y, Bao X. Effects of uric acid on endothelial dysfunction in early chronic kidney disease and its mechanisms. Eur J Med Res. 2013;18(1):26
- Dogan A, Yarlioglues M, Kaya MG, et al. Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011;20(3):182–187
- Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: Molecular mechanisms and clinical implications. J Cardiol. 2012;59(3):235–242
- Waring WS, McKnight JA, Webb DJ, Maxwell SR. Lowering serum urate does not improve endothelial function in patients with type 2 diabetes. Diabetologia. 2007;50(12):2572–2579
- Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int. 2004;65(6):2380–2389
- Liu WC, Hung CC, Chen SC, et al. Association of hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2012;7(4):541–548
- Suliman ME, Johnson RJ, García-López E, et al. J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis. 2006;48(5):761–771
- Wen CP, David Cheng TY, Chan HT, et al. Is high serum uric acid a risk marker or a target for treatment? Examination of its independent effect in a large cohort with low cardiovascular risk. Am J Kidney Dis. 2010;56(2):273–288
- Navaneethan SD, Beddhu S. Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant. 2009;24(4):1260–1266
- Kanbay M, Yilmaz MI, Sonmez A, et al. Serum uric acid independently predicts cardiovascular events in advanced nephropathy. Am J Nephrol. 2012;36(4):324–331
- Neri L, Rocca Rey LA, Lentine KL, et al. Joint association of hyperuricemia and reduced GFR on cardiovascular morbidity: A historical cohort study based on laboratory and claims data from a national insurance provider. Am J Kidney Dis. 2011;58(3):398–408
- Chung W, Kim AJ, Ro H, Chang JH, Lee HH, Jung JY. Hyperuricemia is an independent risk factor for mortality only if chronic kidney disease is present. Am J Nephrol. 2013;37(5):452–461
- Terawaki H, Nakayama M, Miyazawa E, et al. Effect of allopurinol on cardiovascular incidence among hypertensive nephropathy patients: The Gonryo study. Clin Exp Nephrol. 2013;17(4):549–553
- Lee CT, Chua S, Hsu CY, et al. Biomarkers associated with vascular and valvular calcification in chronic hemodialysis patients. Dis Markers. 2013;34(4):229–235
- Antunovic T, Stefanovic A, Ratkovic M, et al. High uric acid and low superoxide dismutase as possible predictors of all-cause and cardiovascular mortality in hemodialysis patients. Int Urol Nephrol. 2013;45(4):1111–1119
- Lobo JC, Stockler-Pinto MB, da Nóbrega AC, Carraro-Eduardo JC, Mafra D. Is there association between uric acid and inflammation in hemodialysis patients? Ren Fail. 2013;35(3):361–366
- Cohen SD, Kimmel PL, Neff R, Agodoa L, Abbott KC. Association of incident gout and mortality in dialysis patients. J Am Soc Nephrol. 2008;19(11):2204–2210
- Lee SM, Lee AL, Winters TJ, et al. Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am J Nephrol. 2009;29(2):79–85
- Fourtunas C. Perceptions of gout (podagra) during the Byzantine era, with a special focus on a poem by Michael Psellus. J Nephrol. 2013;26(Suppl. 22):110–112
- Xia X, He F, Wu X, Peng F, Huang F, Yu X. Relationship between serum uric acid and all-cause and cardiovascular mortality in patients treated with peritoneal dialysis. Am J Kidney Dis. 2014;64(2):257–264
- Feng S, Jiang L, Shi Y, et al. Uric acid levels and all-cause mortality in peritoneal dialysis patients. Kidney Blood Press Res. 2013;37(2--3):181–189
- Dong J, Han QF, Zhu TY, et al. The associations of uric acid, cardiovascular and all-cause mortality in peritoneal dialysispatients. PLoS One. 2014;9(1):e82342
- Mazali FC, Mazzali M. Uric acid and transplantation. Semin Nephrol. 2011;31(5):466–471
- Einollahi B, Einollahi H, Nafar M, Rostami Z. Prevalence and risk factors of hyperuricemia among kidney transplant recipients. Indian J Nephrol. 2013;23(3):201–205
- Alpay N, Ozkok A, Caliskan Y, et al. Influence of conversion from calcineurin inhibitors to everolimus on fibrosis, inflammation, tubular damage and vascular function in renal transplant patients. Clin Exp Nephrol. 2014 [Epub ahead of print]. doi: 10.1007/s10157-014-0939-4
- Meier-Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Uric acid levels have no significant effect on renal function in adult renal transplant recipients: Evidence from the symphony study. Clin J Am Soc Nephrol. 2009;4(10):1655–1660
- Gores PF, Fryd DS, Sutherland DE, Najarian JS, Simmons RL. Hyperuricemia after renal transplantation. Am J Surg. 1988;156(5):397–400
- Akgul A, Bilgic A, Ibis A, Ozdemir FN, Arat Z, Haberal M. Is uric acid a predictive factor for graft dysfunction in renal transplant recipients? Transplant Proc. 2007;39(4):1023–1026
- Akalin E, Ganeshan SV, Winston J, Muntner P. Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation. 2008;86(5):652–658
- Gerhardt U, Grosse Hüttmann M, Hohage H. Influence of hyperglycemia and hyperuricemia on long-term transplant survival in kidney transplant recipients. Clin Transplant. 1999;13(5):375–379
- Armstrong KA, Johnson DW, Campbell SB, Isbel NM, Hawley CM. Does uric acid have a pathogenetic role in graft dysfunction and hypertension in renal transplant recipients? Transplantation. 2005;80(11):1565–1571
- Bandukwala F, Huang M, Zaltzman JS, Nash MM, Prasad GV. Association of uric acid with inflammation, progressive renal allograft dysfunction and post-transplant cardiovascular risk. Am J Cardiol. 2009;103(6):867–871
- Min SI, Yun IJ, Kang JM, et al. Moderate-to-severe early-onset hyperuricaemia: A prognostic marker of long-term kidney transplant outcome. Nephrol Dial Transplant. 2009;24(8):2584–2590
- Choi JY, Kwon OJ. The association between serum uric acid levels at 3 months after renal transplantation and the graft outcome in living donor renal transplantation. Transplant Proc. 2013;45(4):1548–1552
- Huang Y, Li YL, Huang H, Wang L, Yuan WM, Li J. Effects of hyperuricemia on renal function of renal transplant recipients: A systematic review and meta-analysis of cohort studies. PLoS One. 2012;7(6):e39457
- Weng SC, Shu KH, Tarng DC, et al. Uric acid is highly associated with kidney allograft survival in a time-varying analysis. Transplant Proc. 2014;46(2):505–510
- Hart A, Jackson S, Kasiske BL, et al. Uric acid and allograft loss from interstitial fibrosis/tubular atrophy: Post hoc analysis from the angiotensin II blockade in chronic allograft nephropathy trial. Transplantation. 2014;97(10):1066–1071
- Saglam F, Celik A, Sarioglu S, et al. Hyperuricemia influences chronic cyclosporine nephropathy. Transplant Proc. 2008;40(1):167–170
- Boratyńska M, Karbowska A, Klinger M. The effect of hyperuricemia on endothelial biomarkers and renal function in kidney allograft recipients. Transplant Proc. 2010;42(10):4074–4077