Abstract
Objective: To assess the efficacy of thyroid hormone replacement therapy for nephrotic syndrome (NS) patients associated with euthyroid sick syndrome (ESS). Materials and methods: The Cochrane library, ISI, Ovid, PubMed, Chinese Biomedicine Database were searched, and reference list of relevant articles were selected. Randomized controlled trials (RCTs) or quasi-RCTs with thyroid hormone replacement on NS patients associated with ESS were included in this analysis. Results: Six trials (329 participants) were included. Meta-analysis showed that thyroid hormone replacement therapy can significantly increase the completely remission rate [OR = 3.04, 95% confidence interval (CI): 3.04–1.88, p < 0.00001] and total response rate (OR = 4.63, 95% CI: 2.46–8.71, p < 0.00001) of NS patients associated with ESS. No side effect was observed during the follow-up period. There was no obvious publication bias in the mete-analysis studies. Conclusions: Thyroid hormone replacement therapy significantly increases the remission of ESS in patients with NS.
Introduction
Euthyroid sick syndrome (ESS), which is also named nonthyroidal illness syndrome (NTIS) or low triiodothyronine (T3) syndrome, is characterized by decreased serum T3 and thyroxin (T4) levels in critical or noncritical illness, accompanying with increased reverse T3 (rT3) and no significant increase in thyroid-stimulating hormone (TSH).Citation1 It is usually not caused by the thyroid or pituitary gland diseases, but probably a response to the underlying illness,Citation2,Citation3 including severe infections,Citation4,Citation5 trauma,Citation6 major surgery,Citation7,Citation8 myocardial infarction,Citation9 inflammatory conditions,Citation10,Citation11 starvationCitation12 and kidney diseases.Citation13 In most cases, patients with ESS have no peculiar or obvious symptoms, thus ESS might be neglected by doctors. Studies showed that ESS is not uncommon in hospitalized patients, especially patients in the intensive care units,Citation14,Citation15 and it may be a sign of a poor prognosis and may contribute to mortality in critical patients.Citation6
It is still a controversy whether thyroid hormone replacement therapy should be used to these patients. Pappa et al. thought that there is no clear evidence of benefit from thyroid hormone replacement and well-designed studies that confirming its efficacy, thyroxine supplementation should not be recommended for the treatment of NTIS.Citation16 While De Groot et al. hold that ESS is a manifestation of hypothalamic–pituitary dysfunction and should be treated with appropriate replacement therapies.Citation17
In this study, we hope to explore the efficacy and safety of thyroid hormone replacement therapy for nephrotic syndrome (NS) patients associated with ESS. We collected related articles extensively, strictly selected eligible studies, evaluated the efficacy of thyroid replacement therapy for NS patients associated with ESS by the method of meta-analysis, so as to provide guidelines for clinics.
Materials and methods
Literature search
By methods of MeSH, keywords and manual retrieval, we searched the Cochrane library, ISI, Ovid, PubMed and Chinese Biomedicine database. The search terms includes NS, thyroxine/thyroxin/levothyroxine/thyroid hormone, ESS, nonthyroid/NTIS and ESS/NTIS. There were no language and publish date restrictions. Related research references were retrieved at the same time.
Inclusion criteria
For inclusion, trials need to satisfy the following criteria: (a) patients definitely diagnosed with NS and ESS, (b) study design should be randomized controlled trials (RCTs) or quasi-RCTs and (c) complete data and clear definition of completely remission (CR) and partially remission (PR).
Exclusion criteria
Trials were excluded when meet one of the following items: (a) no controls, (b) no complete data, (c) data or papers published repeatedly, (d) subjects have a history of thyroid or pituitary diseases and (e) use of drugs that affect the function of pituitary or thyroid.
Study quality
The quality of included studies was assessed by two researchers independently according to the Jadad standard.Citation18 The Jadad scale includes the following items: (a) with or without randomized methods, (b) appropriateness of randomization methods, (c) with or without double-blind methods, (d) appropriateness of blinding methods and (e) with or without records of incidence of withdraw and dropouts and the reason. The Jadad score ranges from 0 to 5 points, with 0–2 belonging to low quality, 3–5 belonging to high quality.
Data extraction and outcome measures
The process of data extraction of included studies was conducted by two researchers independently. If they disagree with the extracted data, it will be solved through discussion or contacting the author for further information. Extracted information contains the first author, year of publication, the side effects, definition of completely and PR, the follow-up time, the number of participants and the number of completely and partially remitted patients in experimental and control groups. CR: urine protein <0.3 g/24 h with normal kidney function, no relevant symptoms. PR: urine protein 0.3–1.0 g/24 h with normal kidney function, no relevant symptoms. Invalid: urine protein >1.0 g/24 h with or without worsening kidney function, no remission of symptoms.
Statistical analysis
Statistical analysis of included studies was performed with RevMan5.0 software provided by the Cochrane collaboration (The Informatics & Knowledge Management Department, CochraneTech). I2 test and Q statistic test were used to examine the heterogeneity, with I2 > 50% or p < 0.10 considered to be significant.Citation19 If I2 > 50% or p < 0.10, the random effects model was used for meta-analyses, otherwise, the fixed effects model was selected. The difference of effects between experimental and control group was expressed as odds ratio (OR), as well as its 95% confidence interval (95% CI). Publication bias was examined by funnel plot method along with meta-analysis.
Results
Studies included in the meta-analysis
Thirteen relevant articles were identified according to the inclusion and exclusion criteria and six articles (329 patients)Citation20–25 were finally included in this study. The flow diagram of selection of articles is shown in , and the characteristics of each study are listed in . All the patients of the six studies were treated with glucocorticoid as a major, with 1 mg/kg·d of prednisone as a starting dose.
Table 1. Characteristics of included studies.
Effects of thyroid hormone replacement therapy on CR rate
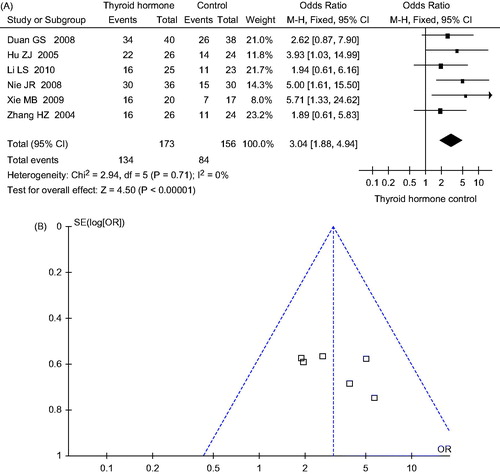
Six studies involved 329 subjects, with 173 in the experimental group and 156 in the control group. After treatment, 134 of 173 subjects in the experimental group and 84 of 156 subjects in control group recovered completely. Meta-analysis shows that there is no significant heterogeneity between six studies (p = 0.71, I2 = 0%), thus, the fixed effects model is selected. The difference of effect between experimental group and control group is statistically significant (OR = 3.04, 95% CI: 1.88–4.94, p < 0.00001), as shown in .
Effects of thyroid hormone replacement therapy on total response rate
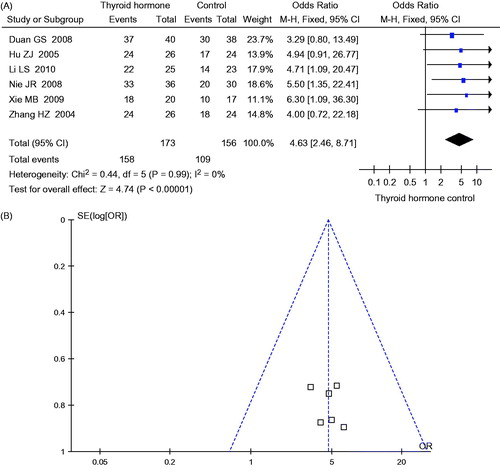
One-hundred fifty-eight of 173 subjects in the experimental group and 109 of 156 subjects in control group responded to the therapy. Meta-analysis shows that there is no significant heterogeneity between six studies (p = 0.99, I2 = 0%). Thus the fixed effects model is selected. The difference of effect between experimental groups and control groups is statistically significant (OR = 4.63, 95% CI: 2.46–8.71, p < 0.00001), as shown in .
Side effects
All six studies have no reports on side effects of experimental and control groups; therefore, we cannot evaluate the potential side effects.
Publication bias
The funnel plots are shown in and . The pattern of funnel plots for CR rate and total response rate are roughly symmetrical for indicating the publication bias.
Discussion
Under physiological conditions, the serum levels of thyroid hormone is closely related to the homeostatic regulation of the hypothalamus–pituitary–thyroid axis in which the hypothalamic thyrotropin-releasing hormone (TRH) stimulates the secretion of TSH by the anterior pituitary, thus resulting in the secretion of thyroid hormone by the thyroid gland.Citation26 The homeostasis regulation of serum thyroid hormone can be disordered in illness or surgical stress conditions, partially resulting in ESS, which with an important influence on the prognosis.Citation15
The mechanism of ESS in NS patients remains unclear. The studies of Fonseca V et al. showed that among the NS patients with urine T4 positive have a lower serum T3 and T4,Citation27,Citation28 suggesting that urinary thyroid hormone loss may play a pivotal role in lower levels of serum T3 and T4. Massive proteinuria may result in loss of thyroid-binding globulin (TBG)Citation29 and furosemide can compete for T4-binding sites on TBG, both contributing to lower total T4 and T3 levels.Citation30 While oxidative stress, psychological pressure and the long-term use of glucocorticoid can inhibit the expression of TSH in pituitary, balancing out the elevated TSH caused by the low levels of serum thyroid hormones, thus making the levels of serum TSH be normal or slightly low.Citation31–33 Yu et al.Citation34 showed that the syndrome was due, in part, to the decreased activity of type I 5′-deiodinase (5′-DI), the hepatic enzyme that converts T4 to T3. By primary culturing of rat hepatocytes, they demonstrated that the expression of 5′-DI was suppressed at the transcriptional level in the condition of ESS, with interleukin-1 and interleukin-6 inhibit the induction of 5′-DI RNA and enzyme activity.
It is still unclear whether ESS represents a physiological protective mechanism, or a damaging maladaptive response, and whether early thyroid hormone administration might improve the prognosis in ESS.Citation35 Actually, the low level of serum thyroid hormone can inhibit the expression of glucocorticoid receptor (GR), with manifestations of steroid resistance.Citation36 The elevated serum thyroid hormone levels after thyroid hormone replacement therapy increases the expression of GR, enhancing the binding of the GR and glucocorticoid, ultimately strengthening the efficacy of glucocorticoid for NS. Thyroid hormone plays a pivotal role in water and sodium homeostasis, renal development and renal hemodynamics.Citation37–39 The renal blood flow was increased after thyroid hormone replacement therapy, promoting the restoration of glomerular basement membrane and the remission of NS.
The meta-analysis shows that thyroid hormone replacement therapy significantly improved the remission of the NS patients with ESS not only in CR rate (OR = 3.04, p < 0.00001) but also in total response rate (OR = 4.63, p < 0.00001) with no records of adverse effects in the process of treatment. No publication bias was detected in this meta-analysis.
As the sample size is relatively small, differences may exist between the results of meta-analysis and that of the actual clinical situations. Therefore, the conclusion should be treated cautiously. For more credible conclusion, studies of multicenter and larger sample sizes are needed.
Declaration of interest
No conflict of interests is declared.
This research was supported by the National Natural Science Foundation of China (no. 81360122/H0518).
This paper is not under consideration elsewhere. None of the paper’s contents has been previously published. All the authors have read and approved the manuscript.
References
- Xu G, Yan W, Li J. An update for the controversies and hypotheses of regulating nonthyroidal illness syndrome in chronic kidney diseases. Clin Exp Nephrol 2014. [Epub ahead of print]. doi: 10.1007/s10157-014-0974-1
- Gibson SC, Hartman DA, Schenck JM. The endocrine response to critical illness: Update and implications for emergency medicine. Emerg Med Clin North Am. 2005;23(3):909–929
- Rolih CA, Ober KP. The endocrine response to critical illness. Med Clin North Am. 1995;79(1):211–224
- Meinhold H, Gramm HJ, Meissner W, et al. Elevated serum diiodotyrosine (DIT) in severe infections and sepsis: DIT, a possible new marker of leukocyte activity. J Clin Endocrinol Metab. 1991;72(4):945–953
- Hamblin PS, Dyer SA, Mohr VS, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab. 1986;62(4):717–722
- Phillips RH, Valente WA, Caplan ES, Connor TB, Wiswell JG. Circulating thyroid hormone changes in acute trauma: Prognostic implications for clinical outcome. J Trauma. 1984;24(2):116–119
- Halabe Cherem J, Nellen Hummel H, Gordon Barabejski F, Chong Martínez BA, Lifshitz Guinzberg A. Thyroid function and abdominal surgery. A longitudinal study. Arch Med Res. 1992;23(3):143–147
- Kniazewski B, Olejek A, Rzempołuch J. Effect of gynecologic abdominal surgery on function of the hypophyseal-thyroid axis. Pol Tyg Lek. 1993;48(27–28):590–594
- Vardarli I, Schmidt R, Wdowinski JM, Teuber J, Schwedes U, Usadel KH. The hypothalamo-hypophyseal thyroid axis, plasma protein concentrations and the hypophyseo-gonadal axis in low T3 syndrome following acute myocardial infarct. Klin Wochenschr. 1987;65(3):129–133
- Park DJ, Cho CS, Lee SH, Park SH, Kim HY. Thyroid disorders in Korean patients with systemic lupus erythematosus. Scand J Rheumatol. 1995;24(1):13–17
- Ormerod AD, How J, Bewsher PD, Reid IW. Effects of widespread dermatitis and topical steroid therapy on thyroid function tests. Dermatologica. 1988;176(5):257–259
- Spalter AR, Gwirtsman HE, Demitrack MA, Gold PW. Thyroid function in bulimia nervosa. Biol Psychiatry. 1993;33(6):408–414
- Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab. 2012;16(2):204–213
- Zargar AH, Ganie MA, Masoodi SR, et al. Prevalence and pattern of sick euthyroid syndrome in acute and chronic non-thyroidal illness—Its relationship with severity and outcome of the disorder. J Assoc Physicians India. 2004;52:27–31
- Di Napoli M, Reda G, Zannoni G, Russo S, Morace G, Vasselli C. The euthyroid sick syndrome. Its incidence and clinical significance in an internal medicine department. Minerva Med. 1994;85:161–165
- Pappa TA, Vagenakis AG, Alevizaki M. The nonthyroidal illness syndrome in the non-critically ill patient. Eur J Clin Invest. 2011;41(2):212–220
- De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin. 2006;22(1):57–86
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1–12
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560
- Xie MB. Curative effect in patients with nephrotic syndrome combined with hypothyroidism. Chin J Health Lab Tech. 2009;19(8):1841–1842
- Duan GS, Cheng XH, Xu WW. Treatment of nephrotic syndrome combined with hypothyroidism. J Clin Nephrol. 2008;8(3):123–124
- Hu ZJ, Li HJ, Niu K, Liu B. Treatment of primary nephrotic syndrome complicated by hypothyroidism. Clin Misdiagn Misther. 2005;18(2):77–79
- Li LS, Zhou ZH, Liu LX. Curative effects of Levothyroxine in patients with primary nephrotic syndrome followed with hypothyroidism. J Med Forum. 2005;31(19):21–23
- Nie JR, Gao QY, Cheng XM. Study the serum levels of thyroid hormone on patients with primary nephritic syndrome and the efficacy of thyroid hormone treatment. Chin J Clin Health. 2008;11(1):33–34
- Zhang HZ, Xu JH, Ren YY. Curative effects of small dose of thyroxine on nephrotic syndrome. The paper collection of the Chinese Medical Association 2004 annual meeting of renal disease, Beijing, China
- Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35(2):159–194
- Fonseca V, Thomas M, Katrak A, Sweny P, Moorhead JF. Can urinary thyroid hormone loss cause hypothyroidism? Lancet. 1991;338(8765):475–476
- Chandurkar V, Shik J, Randell E. Exacerbation of underlying hypothyroidism caused by proteinuria and induction of urinary thyroxine loss: Case report and subsequent investigation. Endocr Pract. 2008;14(1):97–103
- Ito S, Kano K, Ando T, Ichimura T. Thyroid function in children with nephrotic syndrome. Pediatr Nephrol. 1994;8(4):412–415
- Stockigt JR, Lim CF, Barlow JW, et al. Interaction of furosemide with serum thyroxine-binding sites: In vivo and in vitro studies and comparison with other inhibitors. J Clin Endocrinol Metab. 1985;60(5):1025–1031
- Fliers E, Guldenaar SE, Wiersinga WM, Swaab DF. Decreased hypothalamic thyrotropin-releasing hormone gene expression in patients with nonthyroidal illness. J Clin Endocrinol Metab. 1997;82(12):4032–4036
- Vaziri ND. Endocrinological consequences of the nephrotic syndrome. Am J Nephrol. 1993;13(5):360–364
- Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: An update. J Endocrinol. 2010;205(1):1–13
- Yu J, Koenig RJ. Regulation of hepatocyte thyroxine 5′-Deiodinase by T3 and nuclear receptor coactivators as a model of the sick euthyroid syndrome. J Biol Chem. 2000;275(49):38296–38301
- Maréchaud R. Low T3 syndrome. Rev Prat. 1998;48(18):2018–2022
- Leseney AM, Benmiloud M, Befort N, Befort JJ. In vitro evidence that hypothyroidism modifies glucocorticoid receptors. Mol Cell Endocrinol. 1987;52(1–2):1–10
- Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160(4):503–515
- Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23(1):22–26
- Capasso G, De Tommaso G, Pica A, et al. Effects of thyroid hormones on heart and kidney functions. Miner Electrolyte Metab. 1999;25(1–2):56–64