Abstract
Background: Our aim was to evaluate the cost-effectiveness of repeat angioplasty versus new brachiobasilic fistula (BBF) in patients with symptomatic cephalic arch stenosis (CAS). Methods: Patients presenting with symptomatic CAS (n = 22) underwent angioplasty. They were compared to patients undergoing BBF creation (n = 51). Primary outcomes were functional primary arteriovenous fistulae patency at 3, 6 and 12 months. Data were collected on number of interventions, alternative accesses and hospital days for access-related complications. Quality of life was assessed using Kidney Disease Quality of Life-36 scores. Decision tree, Monte Carlo simulation and sensitivity analysis permitted cost-utility analysis. Healthcare costs were derived from Department of Health figures and are presented as cost (£)/patient/year, cost/access preserved and cost/quality of life-adjusted year (QALY) for each of the treatment strategies. Results: Functional primary patency rates at 3, 6, 12 months were 87.5%, 81% and 43% for repeated angioplasty and 78%, 63% and 41% for BBF. The angioplasty cohort required 1.64 ± 0.23 angioplasties/patient and 0.64 ± 0.34 lines/patient. BBF required 0.36 ± 0.12 angioplasties/patient and 1.2 ± 0.2 lines/patient. Patients in the BBF cohort spent an additional 0.9 days/year in hospital due to access-related complications. Mean cost/patient/year in the angioplasty group was £5247.72/patient/year versus £3807.55/patient/year in the BBF cohort. Mean cost per access saved was £11,544.98 (angioplasty) versus £4979.10 (BBF). Average cost per QALY was £13,809.79 (angioplasty) versus £10,878.72 per QALY (BBF). Conclusions: CAS poses a difficult management problem with poor outcomes from conventional angioplasty. Optimal management will depend on patient factors, local outcomes and expertise, but consideration should be given to creation of a new BBF as a cost-effective means to manage this difficult problem.
Introduction
Complications of vascular access are a major cause of morbidity and mortality in patients with end-stage renal disease, accounting for over 20% of hospitalizations in patients on hemodialysis and one-third of all renal bed usage.Citation1 Venous stenosis affects up to 50% of arteriovenous fistulae (AVF) and is the most common cause for vascular access dysfunction.Citation2,Citation3 Cephalic arch stenosis (CAS) (stenosis at the final arch of the cephalic vein before its confluence with the axillary vein) is increasingly recognized as a distinct clinical entity that is implicated in up to three-quarters of dysfunctional brachiocephalic fistulae (BCF).Citation4,Citation5
The treatment of CAS remains a matter of debate.Citation6 Endovascular treatment is complicated by resistance of the stenosis to balloon dilatation, vein rupture and a tendency to restenosis.Citation7 One retrospective series quotes 3, 6 and 12 months primary patency rates of 76%, 42% and 23%, respectively.Citation5 Stenting may have better patency rates but can compromise future vascular access options with occlusion of the basilic system at the confluence of the axillary vein.Citation2,Citation8 Due to these sub-optimal outcomes rates, surgical options for the management of CAS have been explored with some success, including transposition of the cephalic vein to the proximal basilic or axillary veins to bypass the stenosis.Citation9,Citation10 However the majority of patients (12 of 13 in one series) undergoing such transposition procedures developed anastomotic stenoses, albeit with a 92% one-year assisted patency rate.Citation9
These difficulties have led some to consider abandonment of the dysfunctional BCF in favor of an ipsilateral brachiobasilic fistula (BBF). Similar to a cephalic vein transposition, venous drainage of a BBF directly into the axillary vein bypasses the stenotic cephalic arch. A de novo BBF avoids the stenosis commonly seen at the venous anastomoses of transposed cephalic vein AVF.Citation9 Patency rates of BBF in the literature are favorable with 65–70% primary-assisted patencyCitation11,Citation12; however, re-intervention rates remain high.Citation13
The aim of this study was to evaluate the cost-effectiveness of intervention with repeated angioplasty compared to creation of a new BBF in patients with dysfunctional BCF as a result of CAS.
Materials and methods
Institutional board approval was not required for this retrospective study. Approval for data collection was obtained from the local Clinical Effectiveness Department. All patients presenting to our tertiary referral vascular access service at the Western Infirmary, Glasgow, over a three-year period with dysfunctional BCF as a result of CAS were included for analysis. AVF dysfunction included patients with thrombosed fistulae, rising venous pressures, prolonged bleeding times, recirculation and arm swelling.
The vascular access service at the Western Infirmary serves a population of approximately 650 hemodialysis patients in the West of Scotland. Sixty-eight percent of prevalent hemodialysis patients currently dialyze via AVF (52% upper arm). Patients with CAS are primarily treated with angioplasty of the stenotic lesion. These patients undergoing CAS angioplasty were compared to all patients having a de novo BBF created during the same time period with the principal outcome measures being access patency and incremental cost-effectiveness ratio (ICER). All patients were followed up for two years. Data were collected on access outcomes, alternative vascular accesses (including tunnelled central venous catheters [TCVCs]), interventions and hospital admissions to permit calculation of cost for a patient “pathway” rather than individual intervention.
All BBF were created by three consultant surgeons. Pre-operative vein mapping was performed and BBF only created if no alternative autologous option was available. It is our unit’s preference to perform one-stage BBF under general anesthesia with simultaneous transposition and superficialization of the basilic vein; however, there were some cases where the surgical plan was changed intra-operatively or a two-stage procedure was performed at the operating surgeon’s discretion. In the case of one-stage procedures, all patients had a subcutaneous drain placed and were admitted overnight post-operatively. All angioplasties were performed by two consultant radiologists. Routine practice for initial treatment of CAS is angioplasty with 8–10 mm high pressure balloon. Cutting balloon angioplasty or cephalic arch stenting was performed for recurrent lesions. All successful angioplasty was performed as a day-case, with hospital admission access salvage failed and alternative vascular access was required. Clinical surveillance of vascular access was undertaken with monitoring of dialysis access flows and dynamic venous pressures. Pre-emptive intervention was performed on any patient with rising venous pressures or three consecutive venous pressures >150 mmHg.
Clinical outcomes
Primary clinical outcomes were primary assisted AVF patency at 3, 6, 12 and 24 months. Thrombosis rates and secondary patency were also recorded. Additional data were collected on patient survival, the number of interventions required to maintain access patency, alternative vascular accesses (including temporary lines, TCVCs and contralateral AVF) and the additional number of hospital bed days required as a result of vascular access complications (including thrombosis and bacteriemia).
Assessment of costs
Procedural costs and costs for in-patient bed days have been derived from Department of Health National Reference Costs 2011–2012Citation1Citation4 and the Scottish Financial Returns, Information Service Division, Scotland 2011Citation1Citation5 (). These reference costs include both fixed direct costs (those directly related to the service or intervention, e.g. in the case of angioplasty, contrast agents and radiologist/radiographer staffing) and fixed indirect costs (e.g. administration and equipment maintenance). It has been assumed that simple AVF creation or revision will utilize one hour of theatre time and one-stage BBF creation with superficialization which require 1.5–2 hours of theatre time. Costs for consumables and sundries (for example, central venous catheters) have been taken from NHS Greater Glasgow and Clyde purchasing contract agreements. Costs for TCVC and temporary line insertion include staffing costs. For all interventional and surgical procedures, the associated cost of hospitalization has been included based on the actual number of days spent in hospital for each patient. All costs have been calculated in pounds Sterling (£). Only direct healthcare costs have been evaluated.
Table 1. Unit costs of interventions. Interventional radiology and operative costs include all consumables and staffing but not bed days.
Assessment of utility
Quality of life was assessed at baseline, 6, 12 and 24 months using the Short Form-6D (SF-6D) tool. These data were derived from the Kidney Disease Quality of Life-36 (KDQOL-36)Citation16 using the method described by Brazier et al.Citation17 The SF-6D results in a numeric score representing health status.
SF-6D scores were then converted using values derived from a standard gamble method to provide utility weights at baseline, 6, 12 and 24 months.Citation18 A value of 1 represents the best health state and 0 represents death. Utility weights were displayed graphically and connected with straight lines to construct quality-adjusted survival curves. Quality of life-adjusted years (QALYs) were calculated from the area under these curves.
Decision tree
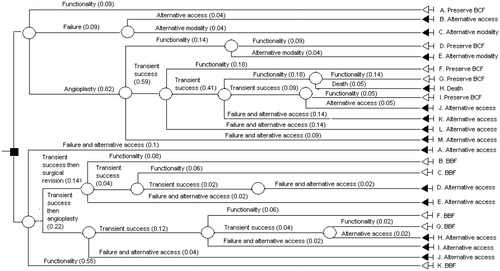
A case-base model was created using a decision tree and populated with outcome data from the two cohorts of patients (CAS angioplasty and BBF creation) using Microsoft Excel 2007 (Microsoft Corp., Redmond, WA) and Tree Plan Toolkit (Microsoft Corp.). The decision tree includes the entire two-year follow-up period. It includes five chance nodes resulting in a total of four possible scenarios (functional patency; transient functionality then recurrent problems; failure with alternative vascular access; and failure with alternative renal replacement therapy [RRT] modality) (). These scenarios include functional AVF without further intervention; successful intervention after initial failure and unsuccessful intervention with change in access modality or RRT. These four scenarios represented five possible health state outcomes at the end of the decision tree (ongoing hemodialysis via original BCF; hemodialysis via de novo BBF; alternative vascular access (either TCVC or AVG); alterative RRT; and death.
Cost-utility analysis
Costs were calculated in pounds Sterling (£) for each of the two treatment groups: cost per patient per year; cost per access preserved per year; and cost per QALY.
Base-case analysis of the decision tree simulation was performed using Microsoft Excel 2007 (Microsoft Corp.), SensIt (Microsoft Corp.) for sensitivity analysis and RiskSim (Microsoft Corp.) for Monte Carlo simulation as recently described for vascular access by Leermakers et al.Citation19 The patient cohort data was used to assess probability and standard error of probability for scenarios in the decision tree. Results are presented as expected value and 95% confidence interval. Cost-bearing events were assumed to have a standard error of 20% of the total cost price. Cost and effect outcomes have been used to calculate an ICER by dividing the difference in costs over the two-year period by difference in QALYs. This is expressed as incremental costs per QALY gained.
A Monte Carlo simulation with 1000 iterations has been performed to encompass both transition uncertainty as well as cost uncertainty using the bootstrap method. The robustness of the model was assessed using sensitivity analysis with input variability for early patency of BBF and functional patency following angioplasty.
Results
Over a three-year period, 22 patients presented with symptomatic CAS requiring angioplasty. Fifty-one patients had a BBF created over the same time period. Basic demographics of the patient cohorts are listed in . All of the initial angiographies restored AVF patency with no complications. Primary assisted patency rates at 3, 6, 12 and 24 months were 87.5%, 81%, 43% and 43%, respectively for repeated CAS angioplasty and 78%, 63%, 41% and 41% following creation of a new BBF. The early failure rate of BBF was 9.8% (n = 5). The repeated angioplasty group required more angioplasties per patient but fewer TCVCs than the BBF creation group (1.64 ± 0.23 angioplasties/patient versus 0.36 ± 0.12 angioplasties/patient; p < 0.01 and 0.64 ± 0.34 TCVCs/patient versus 1.2 ± 0.2 TCVCs/patient; p < 0.01, respectively). Patients in the CAS angioplasty cohort spent an additional 2.6 ± 0.9 days/patient/year in hospital as a result of access related complications compared to an additional 3.5 ± 0.8 days/patient/year in hospital as a result of access-related complications (including bed days for initial access creation) in the BBF creation cohort (p = 0.21) ().
Table 2. Comparison of CAS angioplasty and BBF creation.
Decision tree analysis
The decision tree analysis () showed that 27 of 36 CAS angioplasties (75%) restored patency of the BCF at least transiently. Twenty-eight of 51 BBF (54.9%) had primary functional patency. Forty of 51 BBF (78.4%) achieved primary-assisted patency. At two years follow-up, 78.4% (n = 40) of the BBF and 40.9% (n = 9) of the BCF with CAS angioplasty were still being used for dialysis. One patient had died with a functioning BCF. outlines the proportions of patients in each state at various time points throughout the two year follow-up period. The proportion of patients ending up in different scenarios, together with the associated costs associated with that scenario, is listed in .
Figure 2. Proportion of patients in every state of the decision tree model at various time points for CAS angioplasty (top) and new BBF creation (bottom).
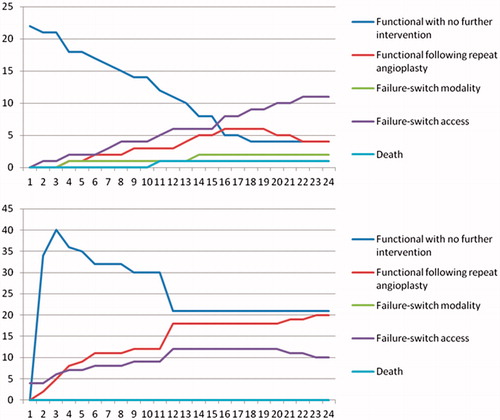
Table 3. Probabilities and costs of scenarios in decision tree.
Base-case analysis
In the angioplasty cohort, the mean cost per patient per year was £5247.72 (£4198.18–£6297.26) compared to £3807.55 (£3046.04–£4569.06) per patient per year in the BBF cohort (p < 0.01) (difference: £1440.17 (£1152.14–£1728.20)). Similarly, the mean cost per access salvaged was lower in the BBF cohort than the angioplasty cohort (£4979.10 (£3983.30–£5974.92) versus £11544.98 (£9235.98–£13853.98), respectively (p < 0.001)). Mean life years during the study period were 2.0 in the new BBF group and 1.98 in the angioplasty group. Health-related quality of life was poor and did not differ significantly between the two groups at any of the measured time intervals (utility weight 0.35 in the angioplasty group versus 0.38 in the BBF group at 1one year). The average cost per QALY was £13,809.79 (£11047.83–£16571.75) in the repeated angioplasty group compared to £10,878.72 per QALY (£8702.98–£13054.46) in the new BBF group (difference: £2931.07 (£2344.85–£3517.29). The calculated ICER (i.e., cost saving of creating a new BBF over angioplasty for every additional QALY) was £4492.13/month.
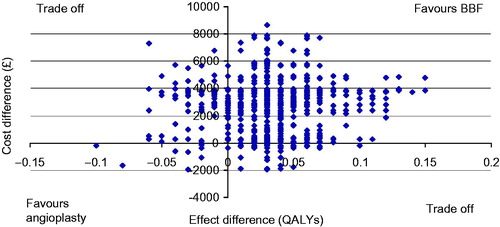
outlines the results of the Monte Carlo simulation on the cost-effectiveness plane. A point in the upper right quadrant indicates in favor of BBF, a point in the lower left quadrant is in favor of angioplasty and both upper left and lower right represent a trade-off. The probability of BBF being less costly and more effective than angioplasty was 93.6%.
Figure 3. Monte Carlo simulation (bootstrap method) of the cost-effectiveness plane for new BBF creation in patients with CAS. The x-axis indicates the difference in QALYs with de novo BBF creation compared to CAS angioplasty, while the y-axis indicates the difference in costs over a two year period for BBF compared to CAS angioplasty. Each point represents a possible outcome given the uncertainty. A point in the upper right quadrant indicates in favor of BBF (with BBF having better QALY at a lower cost than angioplasty); a point in the lower left quadrant indicates in favor of angioplasty (with BBF having worse patency at a higher cost than angioplasty) and both upper left and lower right indicate a trade-off (with worse patency at lower cost or better patency at higher cost).
Sensitivity analysis
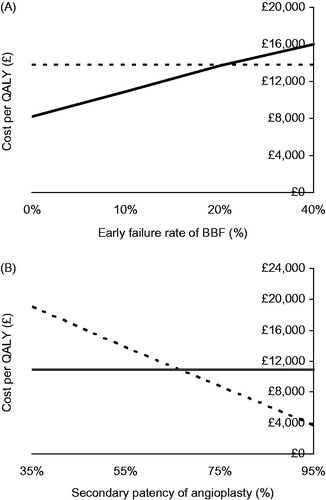
Sensitivity analysis outcomes are shown in . The base-case analysis showed early failure rates of BBF to be 9.8%. Sensitivity analysis of BBF maturation revealed that cost/QALY would be similar between groups if early BBF failure rate increased to 20.2% (). Sensitivity analysis for the success of angioplasty at restoring patency was also evaluated (). Secondary patency of CAS angioplasty was 54.5% in the base-case analysis. Increasing the success of angioplasty to 69.2% resulted in similar cost-utility to BBF.
Discussion
The success of any intervention for vascular access is dictated by optimizing patency rates and minimizing complications. Our aim was to evaluate a method that might permit a rational approach for decision making in comparing the relative benefits of two sub-optimal solutions. We have shown that, whilst patients with a new BBF spent 0.9 additional days in hospital/year as a result of vascular access-related complications, cost-utility analysis favored a de novo BBF rather than repeated angioplasty with a cost-saving of £2931.07 per QALY. More interestingly, the model also allows real-time adjustment based on treatment outcomes, e.g., early failure rates of BBF, would need to double or one-year secondary patency from angioplasty would need to increase to 70% for angioplasty to prove cost-effective over creation of a new BBF.
CAS is associated with significant morbidity and financial costs. Within our hemodialysis population of 650 patients, CAS alone costs £115,500 annually. Moreover, the mean KDQOL-36 score of patients with CAS in this study was significantly lower than in other studies of patients with renal failure,Citation20,Citation21 particularly in the “burden of disease” sub-category with patients believing that they spent “too much time dealing with kidney disease”. Patients in our cohort had average quality of life utility weight of 0.35 per year compared to 0.52 per year quoted by Alehagen et al. for patients on hemodialysis.Citation22 This disparity likely reflects the significant impact that poor vascular access can have on quality of life in patients with CAS, necessitating recurrent admissions and interventions.
Symptomatic CAS was recognized in 3.3% of our total hemodialysis population during the two year follow-up period. There may be a higher underlying rate of CAS in the population (other authors reporting that CAS affects three-quarters of dysfunctional BCF and 15% of dysfunctional AVFCitation4), but only symptomatic patients were investigated in this study. Given the relatively low prevalence of CAS, a randomized controlled trial comparing angioplasty versus BBF would not be feasible in a single centre, and a multicentre trial with varying outcome rates for intervention may not be applicable locally. A cost-effectiveness analysis of local outcomes and follow-up data is a good basis for planning treatment where uncertainty exists between two alternative treatments, neither of which has clear advantage. Our study design permits intelligent prediction of outcomes and subsequent cost-utility analysis based on local prospective data.
The study was designed to answer a very simple question: in patients with BCF and CAS, is it better to repeatedly angioplasty the CAS or create a new BBF? It is recognized that this is a gross oversimplification of the true clinical situation and the complexities of vascular access (different access outcomes at different sites and in different patient groups etc.). It may actually be that it is more cost-effective to abandon ipsilateral access completely and create a radio-/brachio-cephalic fistula in the contralateral arm, with lower operative burden, better patencies and lower associated costs.Citation23 Alternative treatment strategies for CAS have also been proposed. There is some evidence to support additional benefit of cutting balloon angioplastyCitation24 or flow reduction surgery.Citation25 Others have developed experience of cephalic vein transposition for patients with CAS.Citation9,Citation10 The option to create a conduit, which can be cannulated immediately (avoiding the complications and costs associated with TCVCs) is attractive, however, published series demonstrate a high rate of outflow stenoses at the venous anastomosis. The costs of re-intervention need to be offset against any additional gains. Our cost-utility model could easily be adapted by other centers offering these alternative techniques to permit local, unit-specific cost-utility analysis for the treatment of CAS.
To our knowledge, this is the first study comparing costs and effects of vascular access intervention in patients with CAS. Nevertheless, this study has some limitations. First, as already intimated, the data used to populate the decision tree represents local outcomes from a single center. The sensitivity analysis highlights that relatively moderate differences in the outcomes from interventions will significantly alter the balance of cost and effect. Outcomes following angioplasty of CAS in our patient cohort are comparable to other series in the literature. For example, Rajan et al. found primary patency rates of 76%, 42% and 23% at 3, 6 and 12 months in their series of 117 patients undergoing angioplasty of CAS,Citation7 which is comparable to the angioplasty outcomes in our institution. The patency rates of BBF in our cohort are perhaps slightly poorer than those quoted elsewhere in the literature. Hossny reports a 5.7% early failure rate (9.8% in our cohort) and 84.2% secondary patency rate at 12 months.Citation26 These differences likely reflect local case selection. Patients in out cohort only had BBF created if there were no other upper limb native access, with 60.8% having the BBF created as a secondary or tertiary access procedure. Outcomes of second and subsequent AVF are known to be poorer.Citation27 Similarly, 75% of patients in our cohort had a one-stage BBF with transposition. Variations in practice with two-stage procedures and the need for additional operative transposition or superficialization will alter the cost-utility ratio. Second, it must be emphasized that the cohort of patients used to populate the BBF probabilities model did not have CAS. It is current local practice to manage all patients with symptomatic CAS with angioplasty, therefore the BBF cohort represent all the patients having BBF created over the same two-year time period as those undergoing CAS angioplasty. Patients in the BBF cohort had comparable basic demographics to the CAS angioplasty cohort (including age, length of time on hemodialysis and number of previous vascular accesses), and we have no reason to suspect the outcomes of BBF in patients with CAS should differ significantly from the standard population. Third, the study only evaluates direct hospital-related costs and does not evaluate societal impact. However, it is clear from the burden of interventions and the additional bed days utilized by this patient population that there is significant impact of complications and hospitalization beyond those directly related to healthcare costs. By recording the true number of bed days spent per patient rather than arbitrarily assigning an estimated number of bed days for each interventional procedure, this cost-utility analysis may more accurately evaluate the burden of hospitalization than other similar studies. Nevertheless, it was not possible to calculate associated treatment costs of complications, e.g. antibiotics for line infection which occurred during each admission. Finally, cost-utility analysis aims to determine equilibrium where cost and benefit break even for any given population. It does not necessarily consider the best option for the individual patient. It may be that there are adverse anatomical features of the stenosis, which will reduce the success of angioplastyCitation24; poor arterial inflow to the hand increasing the risk of steal from a BBF; an elderly patient with short life expectancy who may benefit from short-term preservation of a BCF with angioplasty rather than awaiting maturation of a BBF; or contralateral central vein stenosis necessitating the aggressive preservation of any patent vascular access. It is hoped that future models may be able to adjust for individual patient characteristics as assisted in personalized cost-effective decision making.
In conclusion, we have demonstrated that CAS is a difficult condition to treat and associated with considerable morbidity. The success of conventional angioplasty is normally short-lived and requires frequent re-intervention. Creation of a de novo BBF demonstrates a favorable cost-utility profile compared to angioplasty in the medium-term. Therefore, as a population-based cost-effective strategy, we would advocate angioplasty of CAS as a bridge to new AVF creation and suggest looking towards creation of a new AVF in patients diagnosed with CAS who have alternative autologous access. However, we caution against the risk that individualized care is compromised by a “one size fits all” approach. Every case must continue to be considered on merit to find a personalized vascular access solution.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
E.L.A.’s salary is funded by Darlinda’s Charity for Renal Research.
References
- Rayner HC, Pisoni RL, Bommer J, et al. Mortality and hospitalization in hemodialysis patients in five European countries: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2004;19:108–120
- Shawyer A, Fotiadis N, Namagondlu G, et al. Cephalic arch stenosis in autogenous hemodialysis fistulas: Treatment with the viabahn stent-graft. Cardiovasc Intervent Radiol. 2013;36:133–139
- Tessitore N, Lipari G, Poli A, et al. Can blood flow surveillance and pre-emptive repair of subclinical stenosis prolong the useful life of arteriovenous fistulae? A randomized controlled study. Nephrol Dial Transplant. 2004;19:2325–2333
- Hammes M, Funaki B, Coe FL. Cephalic arch stenosis in patients with fistula access for hemodialysis: Relationship to diabetes and thrombosis. Hemodial Int. 2008;12:85–89
- Rajan DK, Clark TWI, Patel NK, et al. Prevalence and treatment of cephalic arch stenosis in dysfunctional autogenous hemodialysis fistulas. J Vasc Interv Radiol. 2003;14:567–573
- Heerwagen ST, Lonn L, Schroeder TV, Hansen MA. Cephalic arch stenosis in autogenous brachiocephalic hemodialysis fistulas: Results of cutting balloon angioplasty. J Vasc Access. 2010;11:41–45
- Rajan DK, Bunston S, Misra S, et al. Dysfunctional autologous hemodialysis fistulas: Outcomes after angioplasty – Are there clinical predictors of success? Radiology. 2004;232:508–515
- Shemesh DGI, Zaghal I, Berlowitz D, et al. Angioplasty with stent graft versus bare stent for recurrent cephalic arch stenosis in autogenous arteriovenous access for hemodialysis: A prospective randomized clinical trial. J Vasc Surg. 2008;48:1524–1531
- Kian K, Mishler R, Schon D, et al. Role of surgical intervention for cephalic arch stenosis in the “Fistula First” Era. Semin Dial. 2008;21:93–96
- Chen JC, Jastrzebski J, Taylor DC. Venovenostomy for outflow venous obstruction in patients with upper extremity autogenous hemodialysis arteriovenous access. Ann Vasc Surg. 2005;19:629–635
- Taghizadah A, Dasgupta P, Khan MS, et al. Long-term outcomes of brachiobasilic transposition fistula for hemodialysis. Eur J Vasc Endovasc Surg. 2003;26:670–672
- Oliver MJ, McCann RL, Indridason OS, et al. Comparison of transposed brachiobasilic fistulas to upper arm grafts and brachiocephalic fistulas. Kidney Int. 2001;69:1532–1539
- Lee CH, Ko PJ, Liu YH, et al. Brachiobasilic fistula as a secondary access procedure: An alternative to a dialysis prosthetic graft. Chang Gung Med J. 2004;27:816–823
- Department of Health. National Reference Costs 2011–2012. London: Department of Health. Available at: www.dh.gov.uk/health/2012/11/2011-12-reference-costs/. Accessed August 3, 2013
- Information Service Division, Scotland. Scottish Financial Returns 2011. ISD, Edinburgh. Available at: www.isdscotland.org/Health-Topics/Finance/Costings/File-Listings-2011. Accessed August 3, 2013
- Brazier JE, Harper R, Jones JM, et al. Validating the SF-36 health survey questionnaire: New outcome for primary care. BMJ. 1992;305:160–164
- Brazier JE, Roberts J, Deveril M. The estimation of a preference based measure of health from the SF-36. J Health Econ. 2002;21:271–292
- Kharroubi S, Brazier JE, Roberts JR, et al. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ. 2007;26:597–612
- Leermakers JJPM, Bode AS, Vaidya A, et al. Cost effectiveness of Vascular Access for Hemodialysis: Arteriovenous fistulas versus arteriovenous grafts. EJVES. 2013;45:84–92
- Lowrie EG, Curtin RB, LePain N, Schatell D. Medical Outcomes Study Short Form-36: A consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41:1286–1291
- Mujais SK, Storey K, Brouillette J, et al. Health-related Quality of Life in CKD Patients: Correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–1301
- Alehagen U, Rahmqvist M, Paulsson T, Levin L-A. Quality-adjusted life year weights among elderly patients with heart failure. Eur J Heart Fail. 2008;10:1033–1039
- Weale AR, Bevis P, Neary WD, et al. Radiocephalic and brachiocephalic arteriovenous fistula outcomes in the elderly. J Vasc Surg. 2008;47:144–150
- Heerwagen ST, Lonn L, Schroder TV, Hansen MA. Cephalic arch stenosis in autologous brachiocephalic hemodialysis fistulas: Results of cutting ballon angioplasty. J Vasc Access. 2010;11:41–45
- Miller GA, Friedman A, Khariton A, et al. Access flow reduction and recurrent symptomatic cephalic arch stenosis in brachiocephalic hemodialysis arteriovenous fistulas. J Vasc Access. 2010;11:281–287
- Hossny A. Brachiobasilic arteriovenous fistula: Different surgical techniques and their effects on fistula patency and dialysis-related complications. J Vasc Surg. 2003;37:821–826
- Dixon BS, Novak L, Fangman J. Hemodialysis vascular access survival: Upper arm native arteriovenous fistula. Am J Kidney Dis. 2002;39:92–101