Abstract
In kidney transplantation, renal ischemia and reperfusion injury was one of the leading factors to the development of renal fibrosis, which was the main cause of graft loss. The fibrogenic changes were associated with the long term inflammation elicited by ischemia and reperfusion injury. In the present study, we investigated the role of the Picroside II, the main active constituents of the extract of picrorrhiza scrophulariiflora roots, in attenuating renal fibrosis in a renal ischemia and reperfusion injury model. We induced ischemia and reperfusion injury in kidneys treated with or without Picroside II. We observed that inflammation and tissue fibrosis were increased in ischemia and reperfusion injury group compared to Picroside II group, however, these changes were significantly decreased by the treatment with Picroside II. We concluded that Picroside II can protect the ischemic kidney against renal fibrosis and its mechanism may be through the inhibition of the long term inflammation.
Introduction
Inflammation is recognized as an important component of renal ischemia and reperfusion (I/R) injury.Citation1,Citation2 And the leukocytes are the key mediators that may fuel inflammatory reactions in renal I/R injury. In early phase of reperfusion, various proinflammatory cytokines increased rapidly, which were induced partly by infiltrating mononuclear and endothelial cells. Renal fibrosis can result from the long-term inflammation elicited by I/R injury, which was one of the important contributors to the graft tubular atrophy and interstitial fibrosis, the main cause of graft loss after the first year of transplantation.Citation3
Some studies have demonstrated that neutrophil activation and the infiltration of CD4+ T-cell could persist 6 weeks after injury,Citation4,Citation5 promoting the development of tissue fibrosis. Meanwhile, renal fibrosis is the major pathological change which drives the kidney diseases to the end stageCitation6 and is the result of an imbalance between the synthesis and the degradation of proteins of the extracellular matrix,Citation7 accompanied by the gradual loss of renal function.Citation8 There are many cytokines participating in the fibrogenic process, such as transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF), osteopontin (OPN), collagen I, vimentin, IL-1 and TNF-α. However, other molecules, such as heme oxygenase 1 (HO-1) and bone morphogenic protein-7 (BMP-7) are considered to inhibit the progress of fibrogenesis that former molecules involved.
Picrorrhiza scrophulariiflora belongs to the plant family, scrophularia. The roots of this plant are of benefit and often used in traditional Chinese medicine for a number of conditions.Citation9 Extracts of the roots contain various terpenoids and glycosidesCitation10 and Picroside II is one of the main active constituents of the extracts. Many published studies have shown that Picroside II has a wide range of pharmacological effects, including anti-inflammatory. However, it has not been demonstrated whether Picroside II could protect renal tissue subjected to I/R injury against fibrogenic process. Therefore, the major purpose of this study was to investigate the role of Picroside II in ischemia-induced renal fibrosis and whether its mechanism was related to the inhibition of long term inflammation.
Materials and methods
Animal preparation
All adult male SD rats (220–250 g) were from the Center of Experimental Animals in Medical College, Wuhan University. This project was approved by the committee of experimental animals of Wuhan University, and the procedures were carried out according to the routine animal-care guidelines. All experimental procedures complied with the Guidelines for the Care and Use of Laboratory Animals. Briefly, rats were anesthetized with pentobarbital (45 mg/kg) and placed on a homeothermic table in order to maintain core body temperature at 37 °C. The midline laparotomy was made and right nephrectomy was performed. After it, the left kidney was subjected to 45 min of ischemia followed by reperfusion 12 weeks.
All animals were divided into three different groups: sham group, I/R group and Picroside II group; each group had 8 rats. In sham group, only the right kidneys were removed. In I/R group, the left kidney vessels were clamped for 45 min followed by reperfusion. And the Picroside II group was performed as described below.
Intervention study
Picroside II (CAS No: 39012-20-9, purity >98%, molecular formula C23H28O13) was purchased from Tianjin Kuiqing Medical Technology Co. Ltd (Tianjin, China). It was diluted into 10 g/L solution with 1 mol/L PBS. Picroside II (10 mg/kg) 250 μL was administrated via tail vein in Picroside II group with a micro-syringe according to the previous study,Citation11 at the end of ischemic 45 min before reperfusion 12 weeks. While those in I/R group and sham group were simultaneously injected 1 mol/L PBS 250 μL. At 12 weeks of reperfusion period, the left kidneys were removed for the following experiments and the blood samples were collected for the detection of blood urea nitrogen (BUN) and creatinine (Cr) levels.
Preservation of kidneys
The left kidney was removed under fully maintained anesthesia. After removal, the kidney was fixed in 10% phosphate-buffered formalin or immediately frozen, and stored at −80 °C for following experiments.
Assessment of renal function
At 12 weeks after I/R in every group, 1 ml blood samples were taken and performed according to directions of the commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The absorbance was measured by spectrophotometer and then the concentrations of BUN and Cr were calculated.
Histologic examinations
After the kidney fixed in 10% phosphate-buffered formalin, it was embedded with paraffin and sectioned at 4-μm thick. The sections were deparaffinized and hydrated gradually, and stained with hematoxylin and eosin (H&E) and Sirius red 0.1% in saturated picric acid, respectively. Morphologic assessments were observed by an experienced renal pathologist who was unaware of the treatments. And the changes of renal fibrosis was evaluated based on calculating the percentage of the affected area fraction as described previously, 10 fields per section at ×200 magnification.
Immunohistochemistry
The expression of α-SMA was conducted by immunohistochemical staining. Briefly, 4-μm sections were deparaffinized, and endogenous peroxidase activity was blocked with 3% hydrogen peroxide at 37 °C for 10 min. Then the sections were treated with 10% normal goat serum in Tris-buffered saline (TBS) for 30 min at 37 °C. Subsequently, they were incubated overnight at 4 °C with anti–actin smooth muscle (α-SMA; Santa Cruz Biotechnology, Santa Cruz, CA). After washing three times with PBS, these sections were incubated with the secondary antibody for 30 min at room temperature, followed by color reagent DAB. In negative control group, the experiments were routinely performed.
Realtime PCR
Total RNA were isolated using Trizol reagent (Invitrogen, Carlsbad, CA) and RNA concentration was obtained by spectrophotometer. Single-stranded cDNA was synthesized using the cDNA synthesis kit (Takara, Kyoto, Japan) according to the procedures. Reverse transcription-polymerase chain reaction (PCR) was performed with the Applied Biosystems SYBR Green mix kit (Applied Biosystems, Foster, CA). The primers used were as follows: TNF-α forward primer 5′′- CTTCTCATTCCTGCTCGTGG-3′, and TNF-α reverse primer 5′-TCCGCTTGGTGGTTTGCTAC-3′ (Gen-Bank accession number NM_012675.3); IL-1β forward primer 5′- ACTATGGCAACTGTCCCTGAAC-3′, and IL-1β reverse primer 5′-GTGCTTGGGTCCTCATCCTG-3′ (Gen-Bank accession number NM_031512.2); IL-10 forward primer 5′-GCAGGACTTTAAGGGTTACTTGG-3′, and IL-10 reverse primer 5′-TCATTCTTCACCTGCTCCACTG-3′ (Gen-Bank accession number NM_012854.2); HO-1 forward primer 5′-GTGACAGAAGAGGCTAAGACCGC-3′, and HO-1 reverse primer 5′-GAAACTGAGTGTGAGGACCCATC-3′ (Gen-Bank accession number NM_012580). β-actin was used as a housekeeping gene. The data were presented as a ratio of genes to β-actin mRNA (sense: 5′-TGCTATGTTGCCCTAGACTTCG-3′ and antisense: 5′-GTTGGCATAGAGGTCTTTACGG-3′ and Gen-Bank accession number NM_031144).
Western blot analysis
Total proteins were extracted, and quantified using Bicinchoninic acid method. Then, equivalent weights of protein (40 μg/Lane) was separated on 10% SDS-PAGE gels and then transferred to nitrocellulose membrane. The membranes were blocked with 5% non-fat milk in TBST buffer and then incubated with the following rabbit polyclonal primary antibodies: TGF-β1 (1:1000 dilution; SantaCruz Biotechnology, Santa Cruz, CA), OPN (1:1000 dilution; Abcam, Cambridge, MA), vimentin (1:1000 dilution; Cell Signaling Technology, Boston, MA), collagen I (1:1000 dilution; Millipore), CTGF(1:1000 dilution; SantaCruz Biotechnology, Santa Cruz, CA) and BMP-7 (1:1000 dilution; SantaCruz Biotechnology, Santa Cruz, CA). Subsequently, after being washed twice with PBS, the membranes were incubated with secondary antibody conjugated with horseradish peroxidase at 1:2000 dilution. Specific bands were visualized by using an enhanced chemiluminescence detection kit. Optical densities were detected using Quantity One software.
Statistical analysis
Data were presented as mean ± SEM. The means of the different groups were compared using one-way ANOVA 3 Student–Newman–Keuls test. Differences were considered statistically significant when p < 0.05.
Results
Renal function
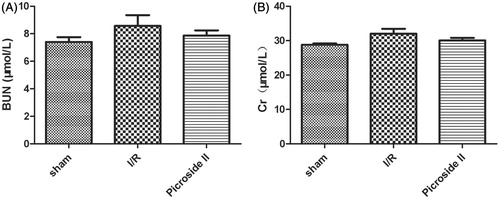
BUN and Cr levels were measured at 12 wks following I/R in rats treated with Picroside II or not. In this model, renal function was not altered significantly and the treatment with Picroside II did not change these results ().
Figure 1. Effects of Picroside II modification on renal function. (A) Effects of Picroside II on the serum BUN concentrations after 45 min of ischemia followed by 12 weeks of reperfusion. (B) Effects of Picroside II on the serum Cr concentrations after 45 min of ischemia followed by 12 weeks of reperfusion (means ± SEM).
Morphologic features
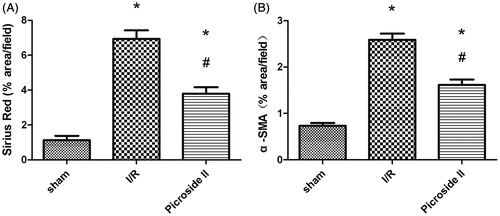
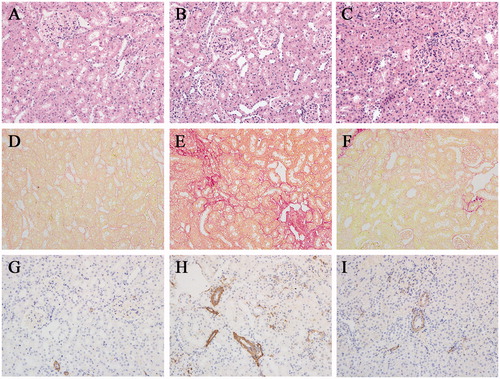
Morphologic features were evaluated by studies, using H&E () and Sirius red staining (). From the serial observation, it indicated that glomeruli sizes tended to be obviously reduced in I/R group and it was common to see the characterization of patchy tubulointerstitial injury and fibrosis, such as infiltration of massive inflammatory cells, tubular dilation or atrophy and interstitial fibrosis. Sirius red staining revealed that rats treated with Picroside II had less fibrosis compared with nontreated rats ().
Figure 2. Picroside II attenuated tissue damage, fibrogenic development and α-SMA expression during I/R. (A, D, G) section from sham-operated rat, (B, E, H) section from rat subjected to I/R, (C, F, I) section from rat subjected to the treatment with Picroside II. (A–C) H&E staining. (D–F) Sirius red staining. (G–I) α-SMA immunohistochemical staining. All H&E, Sirius red and immunohistochemical staining, original magnification ×200.
Immunohistochemistry
In our study, α-SMA was detected by immunohistochemistry staining (). It revealed that α-SMA was rarely found in sham group. But in I/R group, renal tissues were strongly positive for α-SMA expression, mainly in the in the injured renal tubular epithelial cell and tubulointerstitium. Compared with the I/R group, these expressions were ameliorated in Picroside II group.
Realtime PCR analysis
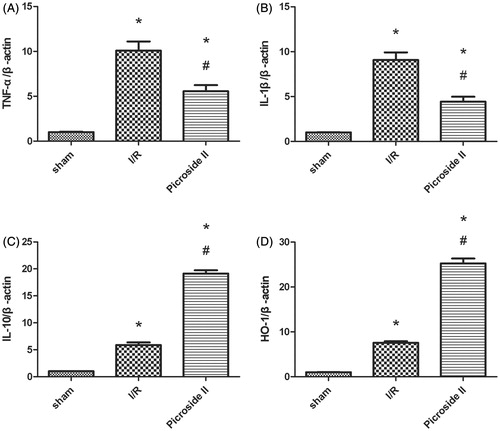
To investigate the mRNA expression of TNF-α, IL-1β, IL-10 and HO-1, we measured their levels by Realtime PCR (RT-PCR). The relative expression of TNF-α, IL-1β, IL-10 and HO-1 to β-actin were shown. The mRNA levels of TNF-α, IL-1β were significantly greater in I/R group than in sham group and Picroside II group, but IL-10 and HO-1 in I/R group were obviously less than those observed in Picroside II group ().
Figure 4. The mRNA level of TNF-α, IL-1β, IL-10 and HO-1 in the kidney. (A) Effects of Picroside II on the mRNA level of TNF-α after 45 min of ischemia followed by 12 weeks of reperfusion. (B) Effects of Picroside II on the mRNA level of IL-1β after 45 min of ischemia followed by 12 weeks of reperfusion. (C) Effects of Picroside II on the mRNA level of IL-10 after 45 min of ischemia followed by 12 weeks of reperfusion. (D) Effects of Picroside II on the mRNA level of HO-1 after 45 min of ischemia followed by 12 weeks of reperfusion. mRNA was standardized for β-actin mRNA (means ± SEM; *p < 0.05 versus sham; # p < 0.05 versus I/R).
Western blot
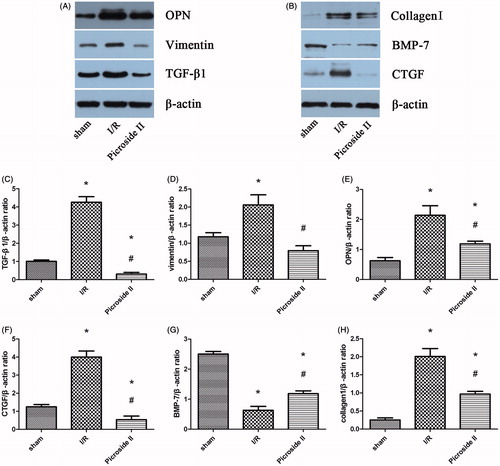
The results of Western blot indicated that the expressions of TGF-β1, OPN, vimentin, collagen I and CTGF were upregulated in I/R group and Picroside II group when compared with sham group. But treatment with Picroside II could attenuate these expression induced by I/R. Compared with sham group, BMP-7 was downregulated in rats subjected to I/R. However, the expression of BMP-7 in Picroside II group was obviously more than that observed in I/R group ().
Figure 5. Representative Western blots showed the effects of Picroside II on TGF-β1, OPN, vimentin, collagen I, CTGF and BMP-7 expression in the kidney after 45 min of ischemia followed by 12 weeks of reperfusion. (A) Representative Western blots showing the effects of Picroside II on TGF-β1, OPN, and collagen I expression. (B) Representative Western blots showing the effects of Picroside II on vimentin, CTGF, and BMP-7 expression. (C) Relative band densities of TGF-β1 to the mean value of the control. (D) Relative band densities of vimentin to the mean value of the control. (E) Relative band densities of OPN to the mean value of the control. (F) Relative band densities of CTGF to the mean value of the control. (G) Relative band densities of BMP-7 to the mean value of the control. (H) Relative band densities of collagen I to the mean value of the control (means ± SEM; *p < 0.05 versus sham; #p < 0.05 versus I/R).
Discussion
I/R injury has elicited an increasing interest of the researchers in its impact on such organs as kidney, liver and heart in recent years. It is the leading antigen-independent factor contributed to the development of chronic allograft loss, which is the foremost cause of graft loss after the first year of kidney transplantation.Citation12 Picroside II is one of the main active constituents of the extracts of picrorrhiza scrophulariiflora pennell and was shown to possess a wide range of pharmacological effects.Citation13,Citation14 However, it was not known about the role of Picroside II in ischemia-induced renal fibrosis. Therefore, the aim of this study was to demonstrate whether Picroside II could have a beneficial effect in prevent the development of renal fibrosis after I/R injury and its possible mechanisms.
Inflammation represents key factor in the occurrence and development of ischemic damage, which is thought to occur secondary to an intense inflammatory response initiated by the infiltration of leukocytes and the production of proinflammatory cytokines after I/R.Citation15 Some studies have demonstrated that a single episode of ischemic injury to kidneys can lead to the maintenance of inflammation and is associated with the development of tissue fibrosis.Citation16 In this study, histomorphometric analyses indicated that the treatment with Picroside II had less infiltration of massive inflammatory cells, tubular dilation or atrophy and interstitial fibrosis.
Treatment with Picroside II could obviously decrease the levels of TNF-α and IL-1β, which are the key proinflammatory cytokines participating in the pathological process of I/R injury. IL-1β has a chemotactic effect, stimulating the proliferation of fibroblasts and possibly the synthesis of extracellular matrix proteins.Citation12 Importantly, the extent of fibrosis could be reduced by the treatment with IL-1β antagonists in rats with glomerulonephritis.Citation17 We also analyzed the expression of IL-10, an anti-inflammatory cytokine, and HO-1, a protective gene. Some studies have demonstrated that the increased expression of IL-10 could reduce intercellular adhesion molecule 1 and selections,Citation18 both of which are responsible for neutrophil rolling in the endothelium, thus protecting the kidney from I/R injury. Therefore, we investigated whether treatment with Picroside II would affect the expression of HO-1 and IL-10. We observed an increase of HO-1 and IL-10 in the ischemic kidneys; however, these increases were significantly higher when the animals were treated with Picroside II. Thus, we can infer that Picroside II had inhibitory effects on the long-term inflammation.
In this study, we analyzed the expression of pro- and anti-fibrotic molecules, such as TGF-β1, vimentin, collagen I, CTGF, OPN, and BMP-7. It has been demonstrated that TGF-β1 is not only one kind of multipurpose cytokines but also a crucial inducer of renal fibrosis. The EMT may be due to TGF-β1 inducing myofibroblast transformation and increasing collagen synthesis.Citation19 In the present study, it could be found that the expression of TGF-β1 was upregulated in I/R group and treatment group, when subjected to I/R, but level changes induced by I/R were attenuated significantly in treatment group. The expression of OPN and collagen I were in accordance with that of TGF-β1. OPN is considered one of the chemokines that attracts macrophages into the injured renal tissueCitation20 and macrophage infiltration correlates with the development of fibrosis.Citation21 So the reduced expression of OPN indicates the reduction of fibrosis and low macrophage infiltration. BMP-7, a member of the TGF-β1 family, regulates TGF-β1 signaling. And CTGF seems to be responsible for some TGF-β1 profibrotic activities.Citation22 In a model of renal fibrosis, it has demonstrated that the increased expression of BMP-7 was associated with the less development of renal fibrosis,Citation23 as well as the inhibition of CTGF. Vimentin, mainly expressed in mesenchymal-derived cells, is a transitional filament protein. It has demonstrated that vimentin expression is increased after I/R in proximal tubular cells.Citation24 In this study, the results indicated that compared with sham group, the expression of CTGF and vimentin showed significant increases in I/R group and treatment with Picroside II could inhibit this increase obviously. BMP-7 expression was downregulated in I/R group, but the level in Picroside II group was significantly upregulated.
In this study, we demonstrate for the first time that Picroside II could protect the ischemic kidney against renal fibrosis. This protective effect may be related with the inhibition of the long-term inflammation. Therefore, these findings reveal the potential role of Picroside II as a future therapy against ischemic renal fibrosis.
Declaration of interest
The authors have no financial conflicts of interest. This study was supported by the Province Natural Science Foundation of Hubei (Grant No. 2013CFB226).
References
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210
- Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–485
- Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333
- Burne-Taney MJ, Yokota N, Rabb H. Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int. 2005;67:1002–1009
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491
- Cook HT. The origin of renal fibroblasts and progression of kidney disease. Am J Pathol. 2010;176:22–24
- Azuma H, Nadeau K, Takada M, et al. Cellular and molecular predictors of chronic renal dysfunction after initial ischemia/reperfusion injury of a single kidney. Transplantation. 1997;64:190–197
- Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456
- Li JX, Li P, Tezuka Y, et al. Three phenylethanoid glycosides and an iridoid glycoside from Picrorrhiza scrophulariiflora. Phytochemistry. 1998;48:537–542
- Zou LC, Zhu TF, Xiang H, et al. New secoiridoid glycosides from the roots of Picrorrhiza scrophulariiflora. Molecules. 2008;13:2049–2057
- Li Q, Li Z, Xu XY, et al. Neuroprotective properties of Picroside II in a rat model of focal cerebral ischemia. Int J Mol Sci. 2010;11:4580–4590
- Feitoza CQ, Goncalves GM, Semedo P, et al. Inhibition of COX 1 and 2 prior to renal ischemia/reperfusion injury decreases the development of fibrosis. Mol Med. 2008;14:724–730
- Cao Y, Liu JW, Yu YJ, et al. Synergistic protective effect of Picroside II and NGF on PC12 cells against oxidative stress induced by H2O2. Pharmacol Rep. 2007;59:573–579
- Smit HF, Kroes BH, van den Berg AJ, et al. Immunomodulatory and anti-inflammatory activity of Picrorrhiza scrophulariiflora. J Ethnopharmacol. 2000;73:101–109
- Du X, Hu X, Wei J. Anti-inflammatory effect of exendin-4 post conditioning during myocardial ischemia and reperfusion. Mol Biol Rep. 2014;41:3853–3857
- Feitoza CQ, Goncalves GM, Semedo P, et al. Inhibition of COX 1 and 2 prior to renal ischemia/reperfusion injury decreases the development of fibrosis. Mol Med. 2008;14:724–730
- Lonnemann G, Shapiro L, Engler-Blum G, et al. Cytokines in human renal interstitial fibrosis. I. Interleukin-1 is a paracrine growth factor for cultured fibrosis-derived kidney fibroblasts. Kidney Int. 1995;47:837–844
- Deng J, Kohda Y, Chiao H, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128
- Stahl PJ, Felsen D. Transforming growth factor-beta, basement membrane, and epithelial-mesenchymal transdifferentiation: Implications for fibrosis in kidney disease. Am J Pathol. 2001;159:1187–1192
- Crisman JM, Richards LL, Valach DP, et al. Chemokine expression in the obstructed kidney. Exp Nephrol. 2001;9:241–248
- Ophascharoensuk V, Giachelli CM, Gordon K, et al. Obstructive uropathy in the mouse: Role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 1999;56:571–580
- Nguyen TQ, Goldschmeding R. Bone morphogenetic protein-7 and connective tissue growth factor: Novel targets for treatment of renal fibrosis? Pharm Res. 2008;25:2416–2426
- Damiao MJ, Bertocchi AP, Monteiro RM, et al. The effects of rapamycin in the progression of renal fibrosis. Transplant Proc. 2007;39:457–459
- Terzi F, Maunoury R, Colucci-Guyon E, et al. Normal tubular regeneration and differentiation of the post-ischemic kidney in mice lacking vimentin. Am J Pathol. 1997;150:1361–1371