Abstract
Background: We investigated the anti-inflammatory and protective effects of concomitant use of dexpanthenol (DXP) and N-acetylcysteine (NAC) induced ischemia/reperfusion (I/R) injury of kidney. Methods: Forty rats were randomly divided into 5 groups. In all groups except for Group 1(Sham), renal arteries bilaterally occluded with vascular clamp for IR injury. Group 1(Sham), received a single dose of 10 mL/kg isotonic saline daily by intraperitoneal (IP) injection for three days. Group 2(IR), received a single dose of 10 mL/kg isotonic saline daily by IP injection for three days. Group 3(IR + NAC), received 300 mg/kg NAC daily by IP injection for three days. Group 4(IR + DXP), received 500 mg/kg DXP daily by IP injection for three days. Group 5(IR + NAC + DXP), received 500 mg/kg DXP and 300 mg/kg NAC daily by IP injection for three days. Serum urea (BUN), creatinine (Cr) and neutrophil gelatinase-associated lipocalin (NGAL, lipocalin 2, siderocalin) levels were measured as kidney function tests. TNF-α levels were measured as inflammatory marker. Tissue sections were evaluated histopathologically under light microscopy. Results: IR + NAC + DXP group received both NAC and DXP before induction of renal I/R and as the biochemical and histopathological data revealed the results of the IR + NAC + DXP group and sham group were similar. Biochemically and histopathologically, combined use of NAC and DXP has better results when each of them used alone. Conclusion: We concluded that concomitant use of DXP and NAC plays a major role against I/R injury and may be useful in acute treatment of I/R induced renal failure.
Introduction
Renal ischemia may occur in many different clinical situations such as sepsis, shock, hydronephrosis, various urological surgical procedures, renal transplantation and cardiopulmonary bypass.Citation1 Ischemia is defined as reduction or cessation of blood flow resulting lack of oxygen and nutrient supply to tissue leading cellular damage. Tissue tries to restore blood flow by reperfusion in order to prevent eventual necrosis, which will occur as the result of ischemia. This effort exacerbates renal dysfunction including paradoxical infiltration of inflammatory cells, production of reactive oxygen species (ROS) and micro vascular damage.Citation2,Citation3
Excessive amounts of ROS play an important role in the pathophysiology of ischemia/reperfusion (I/R) injury.Citation4 Many antioxidant and anti-inflammatory agents such as desferrioxamine,Citation5 ebselen,Citation6 N-acetylcysteine,Citation7,Citation8 dexpanthenol,Citation9,Citation10 pentoxifylineCitation11 and zincCitation12 were studied to prevent or reduce I/R injury of the kidney by oxidative stress and inflammatory response. Dexpanthenol (DXP) and N-acetylcysteine (NAC) are the two drugs used against oxidative stress, inflammatory response and used in many experimental studies individually indicating their tissue protective effects against ROS activitiesCitation8,Citation13, but there is no study including both drugs together.
In our study, we investigated the anti-inflammatory and protective effects of concomitant use of dexpanthenol (DXP), which is an alcoholic analogue of pantothenic acid derivative and N-acetylcysteine (NAC) that is a thiol-compound glutathione precursor antioxidant playing a key role on ROS induced renal I/R injury.
Materials and methods
After obtaining the approval of Animal Research Ethical Committee our study was conducted in Training Hospital Laboratory Animals Research Center. The Guidelines for Animal Research from the National Institutes of Health publication were followed for all experimental procedures.
Animals
Twelve-weeks-old, weighing 250–300 g, 40 male post-pubertal rats of Wistar Albino strains were used in the study. They were housed to include eight rats in each cage and maintained in a temperature- (21 ± 2 °C), humidity- (60 ± 5%) and light- (12:12-hour daily light and dark cycle) controlled room on a standard commercial pellet diet and water ad libitum.
Experimental protocol
Forty rats were randomly divided into 5 groups where n = 8 each group. Blood samples were obtained from each animal 24 hours before the study was initiated for preoperative analysis. After the samples were gathered the study was started with the intraperitoneal (IP) injections.
Groups
Group 1 (Sham surgery group): Without renal artery occlusion received a single dose of 10 mL/kg isotonic saline daily by IP injection for three days.
Group 2 (I/R group): Received a single dose of 10 mL/kg isotonic saline daily by IP injection for three days.
Group 3 (NAC + I/R group): Received 300 mg/kg NAC (Asist ampul®, 300 mg/3 ml Hüsnü Arsan Corp., Istanbul, Turkey) daily by IP injection for three days.Citation8
Group 4 (DXP + I/R group): Received 500 mg/kg DXP (Bepanthene®, 500 mg, Bayer Corp., Istanbul, Turkey) daily by IP injection for three days.Citation1
Group 5 (NAC + DXP + I/R group): Received 500 mg/kg DXP and 300 mg/kg NAC daily by IP injection for three days.Citation1,Citation8
Surgical procedure
Ketamine (100 mg/kg) and chlorpromazine (3–5 mg/kg) were used to anaesthetize the animals 5 minutes before the procedure. Each animal’s lumbar area was shaved with electric clippers and prepared with povidone-iodine (Poviiodeks®, Kim-Pa Corporation, Istanbul, Turkey). Aseptic conditions were maintained in a sterile environment during the surgery. Midline laparotomies were carried out in all groups.
Both renal arteries and veins were explored separately and intravenous (IV) 100 IU/kg heparin (Nevparin®, Mustafa Nevzat Corporation, Istanbul, Turkey) was administered to prevent intravascular coagulation prior to implementation of I/R with vascular clamp. Both arterial and venous blood flow were occluded together through the renal pedicle with atraumatic vascular clamp for the following 60 minutes of ischemia. Adequate vascular occlusion was confirmed by absence of renal artery pulsation and renal parenchymal fade. After 60 minutes of ischemia, by removing the clamps reperfusion period was started for the following 23 hours. Re-initiation of pulsation through the vessels was confirmed visually and palpation of the pulse. After controlling the bleeding, the skin and skin textures were sutured again. In Sham group, same procedure was followed without vascular clamping. At the end of 24th hour, the animals were sacrificed with high doses of anesthesia mixture. Trunk blood sample was extracted for biochemical analysis. Both kidneys were quickly removed, decapsulated and divided into two equal parts longitudinally. From each side of the kidney one of these sections was placed in formaldehyde solution for routine histopathological examination by light microscopy. The other half of the kidney was placed in liquid nitrogen and stored at −70 °C until histopathological analysis.
Biochemical and histopathological analysis
Blood and tissue samples were analyzed in the biochemistry and pathology laboratory. Serum urea (BUN), creatinine (Cr) and neutrophil gelatinase-associated lipocalin (NGAL, lipocalin 2, siderocalin) levels were measured for renal function tests. TNF-α levels were measured as inflammatory marker.
Tissue sections were evaluated for focal necrosis areas in the glomeruli, enlargement of Bowman’s space, tubular dilatation, acute tubular necrosis (ATN), interstitial edema, interstitial inflammation, proteinaceous material accumulation in the tubules and congestion. These histopathological changes were evaluated and graded as follows 0 = none, 1 = mild, 2 = moderate, 3 = severe.
Statistical analysis
Data were analyzed statistically to determine the differences between groups. Minimal amount of animals for each group were calculated for the power of the study to be 0.05 and p value 0.80. Statistical Package for Social Sciences for Windows 15.0 (SPSS Inc., Chicago, IL) program was used to analyze the data obtained from the study. Identifier statistical method (mean, standard deviation, minimum, maximum) was used to analyze the study data. Normal distribution of research data were analyzed with Kolmogorov–Smirnov test. One way ANOVA test was used for comparison of continuous variables between groups. In cases where the variables do not fit with the normal distribution the Kruskal–Wallis test was used. If there was a statistically significant difference between these groups, then Mann–Whitney U test was applied with Bonferroni correction to detect where the difference was oriented. Chi-square test in multiple cells test was used for comparison of histopathological scores between groups. Results were evaluated at 95% confidence interval and p < 0.05 significance level.
Results
No animal died prior or after surgical and treatment procedures.
Biochemical results
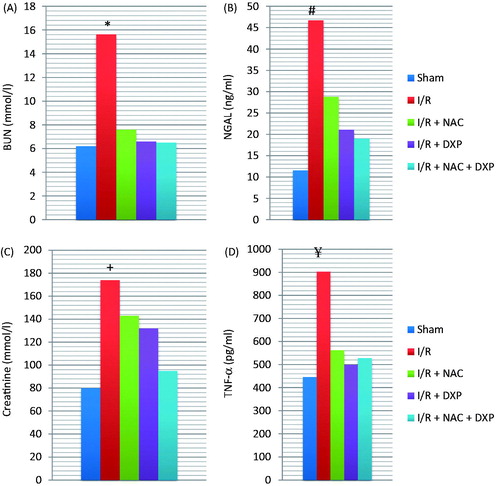
There was no statistical difference between the pre-operative values of each group. Post-operative biochemical data of all groups is presented in . There was no significant difference between the other groups when post-operative BUN values compared except for I/R group (p > 0.05). When post-operative serum creatinine values of sham group and I/R + NAC + DXP group were similar and significantly lower than other groups (p < 0.05). Post-operative NGAL values of sham group and I/R + NAC + DXP group were similar and significantly lower than the other groups (p < 0.05). There was no significant difference between the other groups when post-operative TNF-α values compared except for I/R group (p > 0.05).
Figure 1. Post-operative biochemical values of groups. *Post-operative BUN values of group 2 were significantly higher than other groups; #post-operative NGAL values of group 2 were significantly higher than other groups; +post-operative creatinine values of group 2 were significantly higher than other groups; ¥post-operative TNF-α values of group 2 were significantly higher than other groups.
Histopathological results
Histopathological changes were analyzed for focal necrosis in the glomeruli, range expansion in the Bowman’s capsule, tubular dilatation, acute tubular necrosis (ATN), interstitial edema, interstitial inflammation, proteinaceous material accumulation in the tubules and congestion (). Histopathologic changes scored on a 4 point scale: 0 = no, 1 = mild, 2 = moderate, 3 = severe. Histopathologic scoring of groups are given in . There was significant histopathological damage in group 2 compared to all treatment groups and sham group (p < 0.05). According to data of binary comparison of groups for histopathologic evaluation I/R group and I/R + NAC + DXP were similar (p > 0.05). I/R + NAC + DXP has better histopathologic scoring when compared with I/R + NAC and I/R + DXP groups (p < 0.05).
Figure 2. Histopathological examination under light microscopy. (A) Normal glomerul and tubulus Sham group. (B) Intersitisial inflamation I/R + NAC group. (C) Focal necrosis in glomerul I/R Group. (D) Proteinaceous cast in tubuli I/R Group. (E) Tubuler dilatation I/R + DXP group. (F) Tubuler necrosis I/R group. (G) Congestion I/R + NAC group. (H) Interstitial edema I/R + DXP group. (I) Bowman's capsule dilatation I/R + DXP group.
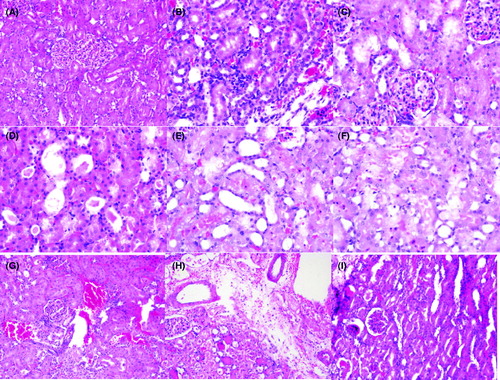
Table 1. Histopathological scoring of groups.
Discussion
In our study, I/R + NAC + DXP group which received both NAC and DXP before induction of renal I/R, had similar biochemical and histopathological results with sham group, which no I/R injury implemented. Biochemically and histopathologically, combined use of NAC and DXP has better results when each of them used alone. As a result, concomitant use of these two drugs before I/R injury comes out with the beneficial effects with decreased renal I/R injury.
I/R, generates excessive amount of oxygen free radicals due to intracellular Ca+2 increase, alteration in mitochondrial oxidative phosphorylation and decreased production of ATP.Citation14 ROS can react with almost all biomolecules in the structure of living organisms and bring out many harmful effects.Citation15 During the ischemia period a small proportion of free radicals are being formed, but on the reperfusion period much larger quantities of free radicals are formed following re-oxygenation and damage leading to lipid peroxidation increases. This process dramatically affects vital organs such as heart, brain and liver so the cases of morbidity and mortality raises.Citation16,Citation17
Enzymatic and non-enzymatic antioxidant mechanisms work against oxidative damage of the tissues. Antioxidant enzymes are two superoxide dismutase (CuZnSOD and MnSOD), glutathione peroxidase (GPx) and catalase (CAT).Citation18,Citation19 Glutathione (GSH) is the most important non-enzymatic endogenous antioxidants. NAC has protective effect against tissue damage caused by free radicals and this effect is realized by increasing the level of GSH or by creating a stable nitrosothiol derivatives. In many experimental models of renal failure NAC was found to be protective against renal I/R injury.Citation6,Citation12,Citation15,Citation20
Dexpanthenol (D-panthenol;(+)-2,4-dihydroxy-N-3-(hidrokdipropil)-3,3 dimethyl butyramide) (DXP) is an alcohol derivative analogue of pantothenic acid (PA) and is oxidized to PA at tissue. Pantothenic acid and its derivates were found to increase reduced glutathione (GSH), coenzyme A (CoA) and adenosine-5-triphosphate (ATP) in the tissues.Citation1,Citation10 As a result, plays an important role against oxidative stress and inflammatory response. Also in many experimental renal failure models dexpanthenol have been shown to have a protective role against the renal I/R injury as well as NAC.Citation9,Citation21
It was previously revealed in experimental models of renal failure that both NAC and DXP individually effective in protection of the tissue. To the best of our knowledge there is no experimental study including both NAC and DXP concomitant use against renal I/R injury. Our study is the first for using both DXP and NAC together before experimental I/R injury model. In accordance with both the biochemical and histopathological results obtained in our experimental renal I/R injury model, it can be indicated that both DXP and NAC have been proven effective against tissue damage. Moreover, the results showed us that concomitant use of these drugs together has better and promising outcomes than their single use. When comparing to NAC group, DXP group reveal significantly better outcome biochemically (NGAL). According to histopathological results (interstitial edema and interstitial inflammation) NAC group came out with better results than DXP group.
Pincemail et al.Citation5 reported that NAC (600 mg/kg) and desferrioxamine (DFO) (50 mg/kg) has positive effects over renal I/R injury in an experimental rabbit model. Especially NAC increases reduced GSH and prevent lipid peroxidation while decreasing ROS. Dexpanthenol was used in some other studies and various of biochemical markers were evaluated (malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and albumin (Alb)) and it was demonstrated that DXP has positive effects on preventing the I/R injury.Citation22,Citation23 Various studies demonstrated the positive tissue protective effects of DXP on liver tissue, testicular tissue following testicular torsion and pulmonary fibrosis respectively.Citation9,Citation21,Citation24
Conclusion
In our study, the beneficial effects of DXP and NAC on renal I/R injury were evaluated for the first time. We concluded that concomitant use of DXP and NAC may be useful in acute treatment of I/R induced renal failure.
Both drugs should be evaluated with further studies for their effects with other I/R models.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Altintas R, Parlakpinar H, Beytur A, et al. Protective effect of dexpanthenol on ischemia-reperfusion-induced renal injury in rats. Kidney Blood Press Res. 2012;36:220–230
- Ricardo SD, Diamond JR. The role of macrophages and reactive oxygen species in experimental hydronephrosis. Semin Nephrol. 1998;18:612–621
- Anaya-Prado R, Toledo-Pereyra LH, Lentsch AB, et al. Ischemia/reperfusion injury. J Surg Res. 2002;105:248–258
- Granger DN, Korthuis RJ. Physiologic mechanisms of post-ischemic tissue injury. Annu Rev Physiol. 1995;57:311–332
- Pincemail J, Defraigne JO, Detry O, Franssen C, Meurisse M, Limet R. Ischemia-reperfusion injury of rabbit kidney: Comparative effects of desferrioxamine and N-acetylcysteine as antioxidants. Transplant Proc. 2000;32:475–476
- Kizilgun M, Poyrazoglu Y, Oztas Y, et al. Beneficial effects of N-acetylcysteine and ebselen on renal ischemia/reperfusion injury. Ren Fail. 2011;33:512–517
- Erdogan H, Fadillioglu E, Yagmurca M, Uçar M, Irmak MK. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: Protective effects of erdosteine and N-acetylcysteine. Urol Res. 2006;34:41–46
- Erbas H, Aydogdu N, Kaymak K. Effects of N-acetylcysteine on arginase, ornithine and nitric oxide in renal ischemia-reperfusion injury. Pharmacol Res. 2004;50:523–527
- Etensel B, Ozkisacik S, Ozkara E, et al. Dexpanthenol attenuates lipid peroxidation and testicular damage at experimental ischemia and reperfusion injury. Pediatr Surg Int. 2007;23:177–181
- Slyshenkov VS, Dymkowska D, Wojtczak L. Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett. 2004;569:169–172
- Oksuz H, Bulbuloglu E, Senoglu N, et al. Re-protective effects of pre- and post-laparoscopy conditioning, zinc, pentoxifylline, and N-acetylcysteine in an animal model of laparoscopy-induced ischemia/reperfusion injury of the kidney. Ren Fail. 2009;31:297–302
- Singh M, Odeniyi DT, Apostolov EO, et al. Protective effect of zinc-N-acetylcysteine on the rat kidney during cold storage. Am J Physiol Renal Physiol. 2013;305:F1022–F1030
- Ceylan H, Yapici S, Tutar E, Ceylan NO, Tarakçıoğlu M, Demiryurek AT. Protective effects of dexpanthenol and y-27632 on stricture formation in a rat model of caustic esophageal injury. J Surg Res. 2011;171:517–523
- Cermik H, Taslipinar MY, Aydin I, et al. The relationship between N-acetylcysteine, hyperbaric oxygen, and inflammation in a rat model of acetaminophen-induced nephrotoxicity. Inflammation. 2013;36:1145–1152
- Seifi B, Kadkhodaee M, Delavari F, Mikaeili S, Shams S, Ostad SN. Pretreatment with pentoxifylline and N-acetylcysteine in liver ischemia reperfusion-induced renal injury. Ren Fail. 2012;34:610–615
- Slusser SO, Grotyohann LW, Martin LF, Scaduto RC Jr. Glutathione catabolism by the ischemic rat kidney. Am J Physiol. 1990;258:F1546–F1553
- Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:1–43
- Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678
- Greene EL, Paller MS. Oxygen free radicals in acute renal failure. Miner Electrolyte Metab. 1991;17:124–132
- DiMari J, Megyesi J, Udvarhelyi N, Price P, Davis R, Safirstein R. N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol. 1997;272:F292–F298
- Slyshenkov VS, Omelyanchik SN, Moiseenok AG, Trebukhina RV, Wojtczak L. Pantothenol protects rats against some deleterious effects of gamma radiation. Free Radic Biol Med. 1998;24:894–899
- Parlakpinar H, Olmez E, Acet A, et al. Beneficial effects of apricot-feeding on myocardial ischemia-reperfusion injury in rats. Food Chem Toxicol. 2009;47:802–808
- van Haaften RI, Haenen GR, Evelo CT, Bast A. Effect of vitamin E on glutathione-dependent enzymes. Drug Metab Rev. 2003;35:215–253
- Ermis H, Parlakpinar H, Gulbas G, et al. Protective effect of dexpanthenol on bleomycin-induced pulmonary fibrosis in rats. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:1103–1110

