Abstract
Purpose: Heavy metals such as mercury can induce the generation of free radicals and oxidative stress which are associated with tissue injury. The present study was designed to evaluate the protective effect of pomegranate seed oil against HgCl2-induced nephrotoxicity. Methods: Twenty-four W/A adult rats were randomly divided into four groups. Group I received corn oil (1 mL/kg). Group II received HgCl2 (5 mg/kg) for 3 days. Group III and IV received PSO 0.4 mL/kg and 0.8 mL/kg, respectively one hour before HgCl2 administration for 3 days. Blood samples were taken by cardiac puncture and used for the measurement of urea and creatinine concentration. Twenty-hour-hour urine samples were collected to measure protein and glucose. The right kidney was fixed in formalin for histological examination and the left kidney was homogenized for measuring malondialdehyde (MDA) and total sulfhydryl groups. Results: Significant elevation of serum creatinine and urea levels as well as urine glucose and protein concentrations, a significant decrease in total thiol content and a significant increase in MDA levels in kidney homogenate samples were observed after administration of HgCl2 as compared with control group. PSO pretreatment resulted in a significant decrease in serum creatinine and urea levels as well as urine glucose and protein concentrations when compared with HgCl2 treated (group II). PSO also significantly reversed the HgCl2-induced depletion in thiol content and elevation in MDA content. Histological studies revealed milder kidney lesions in PSO treated groups (groups III and IV) compared to HgCl2 treated group. Conclusion: Our results suggest that PSO has a protective effect against HgCl2-induced nephrotoxicity in rats.
Introduction
Industrialization in today's world increases the environmental pollutant exposures, such as mercury.Citation1 Previously, HgCl2 was used as disinfectant, laxative, and in the treatment of depression, and parasitic infections. Today, the use of mercury and its compounds has become limited due to its toxicity, but is still used as a laboratory reagent.Citation2,Citation3 Renal toxicity is one of the significant toxicities, among the confirmed cases. This complication occurs mainly in the proximal tubule. The mechanism of Hg-induced nephrotoxicity is not completely understood, but many studies have been found that oxidative stress is an important molecular mechanism for its kidney injury.Citation4 HgCl2 toxicity causes overproduction of reactive oxygen species (ROS) in kidney, leading to oxidative damage and cellular dysfunction.Citation1 Morphological and biochemical studies have shown an increase in the production of peroxides such as hydrogen peroxide in renal tubules, decrease in mitochondrial ATP production in cells, and decrease in the activity of free radical scavenging (systems superoxide dismutase and glutathione peroxidase) involved in HgCl2-induced nephrotoxicity.Citation2,Citation5 The mechanism of this toxicity is mainly related to the effect of HgCl2 on renal antioxidants such as glutathione and finally involvement of ROS in this area.Citation6–9 Considering that oxidative stress play an important role in mercury-induced nephrotoxicity, antioxidants are expected to have protective effect against it.Citation10
Several studies have showed that pomegranate, Punica granatum L., as a fruit native to Middle East, has therapeutic effects as being an antioxidant,Citation11–14 anti-proliferation,Citation15–17 anti-inflammatory,Citation18,Citation19 anti-microbial and anti-cancer agent.Citation20,Citation21 The protective effects of pomegranate seed oil (PSO) against nephrotoxicity induced by gentamicin and Hexachlorobutadiene had also been shown.Citation22,Citation23
The searches for new drug molecules, nowadays, guide the researchers across the globe toward natural products as an alternative source of medicinal compounds.Citation23 This study was designed to evaluate the protective effect of PSO on HgCl2-induced nephrotoxicity in rats.
Materials and methods
Animals
Twenty-four adult male Wistar rats (Animal House, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran), weighing 170–190 g, were used for all experiments. These animals were housed in a pathogen-free facility on a 12-hour light/dark schedule and with ad lib access to food and water. All animal procedures were approved by the University Ethics Committee and were in compliance with National Laws and with National Institutes of Health guidelines for the use and care of laboratory animals.
Chemicals
DTNB (2,2′-dinitro-5,5′-dithiodibenzoic acid), TBA (2-thiobarbituric acid), n-butanol, Na2EDTA (ethylenediaminetetraacetic acid disodium salt), Trizma base [Tris (hydroxymethyl) aminomethane], HCl (hydrochloric acid), KCl (potassium chloride), phosphoric acid (1%), ether, TCA (Trichloroacetic acid) and methanol were purchased from Merck (Darmstadt, Germany). HgCl2 (mercuric chloride) with purity of 95% was obtained from May & Baker (London, England). PSO d = 0.81 g/mL at 25 °C was a kind gift from Urom Narin Co. (Uromeya, Iran).
Experimental design
After acclimatization, animals were randomly divided into four groups (six each) and individually put in the metabolic cages. Group I (control) was treated with corn oil (1 mL/kg). Group II was injected with a single dose of HgCl2 (5 mg/kg). Groups III and IV were treated with PSO (0.4 and 0.8 mg/kg, respectively) 1 h before HgCl2 (5 mg/kg) injection. All procedures were carried out between 10–12 am. All injections last for three days and were performed intraperitoneally. Twenty-four-hour urine samples were also collected for measuring glucose and protein concentrations. Animals were sacrificed 24 h after the last injection of HgCl2 using ether anesthesia and blood samples were taken out by cardiac puncture for measuring the levels of serum urea and creatinine. The left kidneys were homogenized in cold KCl solution (1.5%, pH 7) to give a 10% homogenate suspension and used for biochemical assays.
Biochemical methods
Urea and creatinine measurement
Glucose concentration was estimated by the enzymatic assay (glucose oxidase) and protein concentration was measured by the turbidimetric method.Citation24,Citation25
Urea concentration was determined colorimetrically using Autoanalyzer (Technicon RA-1000, London, England) and urea kit (Man Lab Company, Tehran, Iran). Creatinine concentration was measured by the Jaffe's method.Citation26
Calculating MDA content
The lipid peroxidation level of the kidney tissues was measured by malondialdehyde (MDA), which is the end product of lipid peroxidation and reacts with TBA as a thiobarbituric acid reactive substance (TBARS) to produce a red-colored complex which has peak absorbance at 532 nm.Citation27 Briefly, 3 ml phosphoric acid (1%) and 1 ml TBA (0.6%) were added to 0.5 ml of homogenate in a centrifuge tube and the mixture was heated for 45 min in a boiling water bath. After cooling, 4 ml of n-butanol was added to the mixture, vortexed for 1 min, and centrifuged at 20,000 rpm for 20 min. The organic layer was transferred to a fresh tube and its absorbance was measured at 532 nm. MDA content was calculated from this equation:
Calculation of total thiol groups
Total SH groups were measured using DTNB as the reagent. This reagent reacts with SH groups to produce a yellow-colored complex which has peak absorbance at 412 nm. Accurately1 ml Tris–EDTA buffer (pH = 8.6) was added to 50 μl kidney homogenate in 2 ml cuvettes and absorbance was read at 412 nm against Tris–EDTA buffer alone (A1). Then, 20 μl DTNB reagent (10 mM in methanol) was added to the mixture, and after 15 min (stored in laboratory temperature), the sample absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mm) was calculated from the following equation:Citation22,Citation23
Histological method
The right kidneys were fixed in formalin (10%) for pathological assays and stained by Hematoxylin and Eosin (H & E).
Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey–Kramer post hoc test for multiple comparisons. The p-values less than 0.05 were considered to be statistically significant.
Results
Histological observations
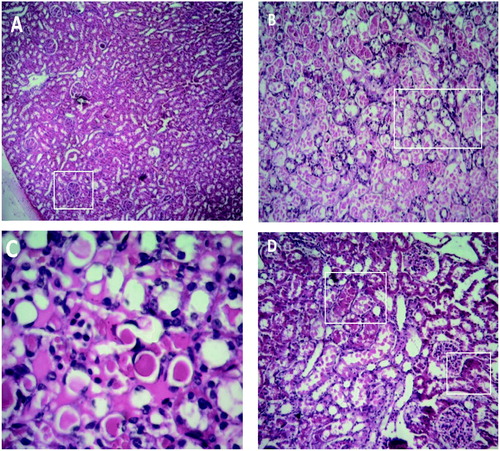
Renal histopathological changes have shown in . As it is shown, there is no evidence of structural changes and tissue damage in control group (1–A). In HgCl2 treated group (1–B), moderate to severe tubular necrosis and atrophy of renal tissue were observed. Structural and morphological changes in pomegranate seed oil-fed groups shown in , respectively. Tubular necrosis rate is at low levels in Group IV and mild level in Group III The hyaline casts in group II compared to experimental groups III and IV were reported as mild (++).
Figure 1. Comparison of structural changes due to injection HgCl2 and PSO between groups. (A) group 1 treated with corn oil, represents a normal rat kidney. All anatomical structures look normal. The box represents glomerulus (×100), (B) group 2 treated with HgCl2 5 mg/kg, severe tubular necrosis and atrophy is observed. All structures (boxed area) have been extensively damaged (×100), (C) group 3 treated with PSO 0.4 ml/kg, followed by HgCl2 injection, mild necrosis have been seen (×400), (D) group 4 treated with PSO 0.8 ml/kg, followed by HgCl2 injection, minimal necrosis have been seen (×100). Tubular necrosis rate is at low levels in Group 4 and mild level in Group 3 (boxed area).
Biochemical results
The amounts of glucose and protein excreted in urine in group II in comparison with control group were significantly elevated. A significant elevation of serum creatinine (1.8-fold) and urea (2.5-fold) was seen (p < 0.05). Total thiol content was decreased after the administration of HgCl2 but it was not significant (49.47 mM vs. 44.3 mM, p > 0.05). HgCl2 caused 2.5% elevation of renal MDA but it was not significant (p > 0.05; ).
Figure 2. Effect of pomegranate seed oil on concentration of urinary glucose (A), urinary protein (B), serum creatinine (C), serum urea (D), renal MDA (E) and total thiol contents (F) in different groups of treated animals. All injections were carried out intraperitoneally. PSO was injected 1 h before HgCl2. Data shown as mean ± SEM (n = 6). *p < 0.05 as compared with HgCl2-treated animals.
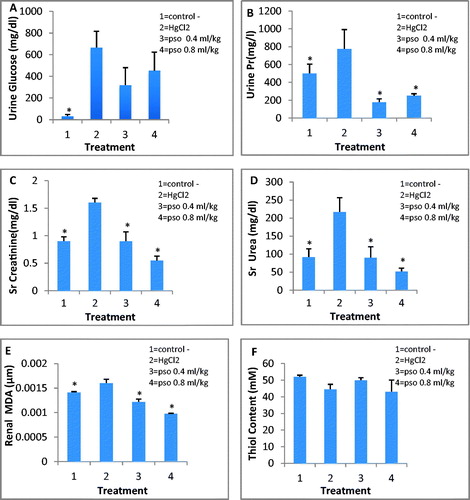
PSO pretreatment resulted in a significant decrease in protein excreted in urine (860 vs. 162 mg/L in group III and 860 vs. 228 mg/L in group IV p < 0.05). Non-significant decrease in glucose (791.6 vs. 218 mg/dL in group III and 791.6 vs. 405.5 mg/dL in group IV p > 0.05) compared with group II. Decrease in serum urea and creatinine in groups III and IV was significant compared with group II (p < 0.05). Serum urea concentrations in group III was 96.8 mg/dL and in group IV was 51.5 mg/dL that in compare with group II (210.8 mg/dL) was significant (p < 0.05). Values for creatinine in group III and IV were 0.98 mg/dL and 0.56 mg/dL, respectively and in comparison with group II (1.51 mg/dL) the differences were significant (p < 0.05). PSO also reversed the HgCl2-induced depletion in total thiol content (10% and 25% increase in group III and IV, respectively) but they were not significant (p > 0.05). Also, it decreased significantly renal MDA (33% and 41% in group III and IV, respectively p < 0.05).
Discussion
One of the most important side effects of mercuric chloride in acute or chronic exposure is renal toxicity.Citation20 The most sensitive part of kidney is proximal tubule. Renal toxicity of mercury is dependent on the destruction time and its density, which can be seen as destruction and vacuolization of microvilli in membrane base and degradation of the cytoskeleton proximal tubules.Citation20,Citation28 The proposed mechanisms of mercuric chloride nephrotoxicity include: (A) Ischemia, decreased renal perfusion and activation of the rennin-angiotensin system, (B) Increased intracellular free calcium and damage to structural proteins, (C) producing antibody in chronic exposure and damage to the glomerular basement membrane, and (D) direct cellular toxicity caused by the activation of cellular oxidative stress, which results in the production of ROS, free radicals, lipid peroxidation, and the destruction and removal of damaged cellular antioxidant enzymes such as glutathione peroxidase and thiol groups, and direct damage to DNA.Citation28
Having a wide range of different antioxidant chemicals, herbal compounds have the ability to inhibit toxicity of exogenous compounds.Citation29 Pomegranate seed oil contains herbal phytosterols (dacosterol and beta sitosterol), hydroxybenzoic acid (gallic, ellagic and their derivatives) and derivatives of glucopyranoside which all have antioxidant and anti-inflammatory effects.Citation23,Citation30 Also, it is considered a rich source of conjugated fatty-acids particularly punicic acid. Moreover, it contains polyphenolic compounds which were considered as important antioxidant and anti-inflammatory compounds.Citation23,Citation31 These compounds show their anti-inflammatory effects through inhibition of pro-inflammatory enzymes and cytokines. In addition, in-vitro studies acknowledged that pomegranate seed oil inhibits tumor cell proliferation.8 In-vivo studies showed that this oil increases the function of B-cells.Citation31
In the present study, mercuric chloride with a dose of 5 mg/kg could induce renal dysfunction. The histological changes along with a significant increase in serum urea and creatinine levels and increased excretion of glucose and protein in the urine are indicators of mercuric chloride toxicity. Direct toxicity of HgCl2 results in increased urinary glucose and protein excretion. Renal toxicity of mercuric chloride has been shown in many previous studies.Citation4,Citation6,Citation10 Decrease in cellular thiol groups and increase in lipid peroxidation after prescribing mercuric chloride, indicate increased oxidative stress and reduced antioxidant enzyme activity which are the main causes of renal toxicity of mercuric chloride. In the present study, pomegranate seed oil pretreatment resulted in a significant and dose-dependent decrease in serum urea and creatinine levels. Decrease in glucose and urine protein excretion was observed as an effect of pomegranate seed oil. This effect was not dose-dependent. The probable cause is shortness of testing process. Likely, chronic treatment of pomegranate seed oil can induce protective effects on renal tubules. Histological changes also revealed that pomegranate seed oil pretreatment resulted in a significant and dose-dependent decrease in the rate of tubular atrophy and necrosis. In the fourth group, which had received 0.8 mL/kg of this oil, toxic mercury effects of necrosis and cellular casts were less than the second and third groups.
In the present study, a significant reduction in lipid peroxidation and increase in the level of thiol contents of cells were observed after using pomegranate seed oil. Malondialdehyde is a stable metabolite of lipid peroxidation caused by oxidative stress in cells. Pomegranate seed oil with doses of 0.4 and 0.8 mL/kg could reduce oxidative stress caused by mercuric chloride. In addition, the amount of thiol content, an important part of the structural proteins and non-protein compounds, plays an important role in the cellular processes such as enzyme activity and restoration of cellular pathways and mechanisms in cell detoxification.Citation32,Citation33 This reduction was offset by the effect of pomegranate seed oil. Impairment of antioxidant enzymes such as glutathione peroxidase and glutathione transferase caused by mercuric chloride, mentioned in the other study,Citation33 together with oxidative stress produced in the glomeruli are the main mechanisms of renal harm.
Pomegranate seed oil contains ellagic acid, an antioxidant compound that removes peroxy radical and prevents Cu2+-induced lipid peroxidationCitation34 by removing Cu2+. On the other hand, punicic acid, the main polyunsaturated fatty acid in pomegranate seed oil, is a strong inhibitor of TNFa-induced priming of ROS production and myeloperoxidase (MPO) release by neutrophils.Citation35 Tissue protection could be the result of the inhibition of ROS/MPO-induced tissue damage.
HgCl2 has very-high affinity for sulfhydryl groups in reduced glutathione and thus it produces oxidative damage by disturbing the prooxidant–antioxidant balance.Citation36 Free radicals including hydroxyl radicals, singlet oxygen, peroxyl radicals and peroxynitrite are able to produce modifications to the DNA bases, strand breakage and various other DNA damages. In addition, ROS can attack protein or lipid molecules (process of lipid peroxidation) and generate intermediates that can react with DNA to form adducts. Malondialdehyde can react with nucleic acid bases to form multiple adducts and causes DNA damage.Citation37 Punicic acid and other ingredients of pomegranate seed oil can react with these processes and reduce oxidative stress.
The possible mechanism concludes hydroperoxide formation and biohydrogenation of conjugated linolenic acids (ClnA). It appears to act as a chain-breaking antioxidant by trapping chain propagating free radicals. Some researchers showed that ClnA could play its antioxidative roles by directly acting on free radicals to terminate the radical chain reaction or chelating transition metals to suppress the initiation of radical formation.Citation38
Conclusion
The results of this study showed that PSO attenuated HgCl2-induced nephrotoxicity. This is supported by the improvement of serum markers of renal function and decrease of proteins and lipids damage, but explanation and mechanism of this protection need further explorations.
Declaration of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This investigation was financially supported by Pharmacological Research Center of Medicinal Plants, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
References
- Deng Y, Xu Z, Liu W, Yang H, Xu B, Wei Y. Effects of lycopene and proanthocyanidins on hepatotoxicity induced by mercuric chloride in rats. Biol Trace Elem Res. 2012;146:213–223
- Goldman LR, Shannon MW. Technical report: Mercury in the environment: Implications for pediatricians. Pediatrics. 2001;108:197–205
- Maillard AP, Fraise PA, Lambert Jean-Yves. Principles and Practice of Disinfection, Preservation and Sterilization. Oxford: John Wiley & Sons; 2007
- Nikolic J, Kocic G, Jevtovic-Stojmenov T. Effect of bioflavonoid lespeflan on xanthine oxidase activity in mercury chloride toxicity. Pharmacologyonline. 2006;3:669–675
- Goering PL, Morgan DL, Ali SF. Effects of mercury vapor inhalation on reactive oxygen species and antioxidant enzymes in brain and kidney are minimal. J Appl Toxicol. 2002;22:167–172
- El-Shenawy SMA, Hassan NS. Comparative evaluation of the protective effect of selenium andgarlic against liver and kidney damage induced by mercury chloride in the rats. Pharmacol Rep. 2008;60:199–208
- Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206
- Mena P, Girones-Vilaplana A, Moreno DA, Girones-Vilaplana C. Pomegranate fruit for health promotion: Myths and realities. Func Plant Sci Biotech. 2011;5(2):33–42
- Agarwal R, Goel SK, Chandra R, Behari JR. Role of vitamin E in preventing acute mercury toxicity in rat. J Environ Toxicol Pharmacol. 2010;29:70–78
- Oda SS, El-Ashmawy IM. Protective effect of silymarin on mercury-induced acute nephro-hepatotoxicity in rats. J Global Veterinaria. 2012;9(4):376–383
- Sharma MK, Sharma A, Kumar M. Spirulina fusiformis provides protection against mercuric chloride induced oxidative stress in Swiss albino mice. Food Chem Toxicol. 2007;45:2412–2419
- Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. “Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum l.) polyphenols after ingestion of a standardized extract in healthy human volunteers”. J Agric Food Chem. 2006;23:8956–8961
- Kulkarni AP, Mahal HS, Kapoor S, Aradhya SM. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J Agric Food Chem. 2007;55(4):1491–1500
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Fuhrman B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation. Am J Clin Nutr. 2000;71:1062–1076
- Hora JJ, Maydew ER, Lansky EP, Dwivedi C. Chemopreventive effects of pomegranate seed oil on Skin tumor development CD1 mice. J Med Food. 2003;6(3):157–61
- Schubert SY, Lansky EP, Neeman I. Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. J Ethnopharmacol. 1999;66(1):11–17
- Seeram NP, Adams LS, Henning SM, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16(6):360–7
- Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D. Heber Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–985
- Esmaillzadeh A, Tahbaz F, Gaieni I, Alavi-Majd H, Azadbakht B. Concentrated pomegranate juice improves lipid profiles in diabetic patients with hyperlipidemia. J Med Food. 2004;7:305–308
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52:113–143
- Stacchiotti1 A, Lavazza A, Rezzani1 R, Borsani1 E, Rodella1 L, Bianchi R. Mercuric chloride-induced alterations in stress protein distribution in rat kidney. J Histol Histopathol. 2004;19: 1209–1218
- Boroushaki MT, Asadpour E, Sadeghnia HR, Dolati K. Effect of pomegranate seed oil against gentamicin – induced nephrotoxicity in rat. J Food Sci Technol. 2012 ; DOI 10.1007/s13197-012-0881-y
- Bouroshaki MT, Sadeghnia HR, Banihasan M, Yavari S. Protective effect of pomegranate seed oil on hexachlorobutadiene-induced nephrotoxicity in rat. Ren Fail. 2010;32:612–617
- Lott JA, Turner K. Evaluation of Trinder's glucose oxidase method for measuring glucose in serum and urine. Clin Chem. 1975;21:1754–1760
- Mc Elderry LA, Tarbit IF, Cassells-Smith AJ. Six methods for urinary protein compared. J Clin Chem. 1982;28:356–360
- Masson P, Ohlsson P, Bjorkhem I. Combined enzymatic – Jaffe's method for determination of creatinine in serum. Clin Chem. 1981;27:18–21
- Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion induced oxidative damage in rats. J Pharm Pharm Sci. 2005;8:387–393
- Wang HS. The effects of mercuric chloride on cultured atlantic spotted dolphin (Stenella plagiodon) renal cells and the role of selenium protection. 2000 ; MSc thesis
- Gurib-Fakim A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93
- Wang RF, Xie WD, Zhang Z, et al. Bioactive compounds from the seeds of Punica granatum (pomegranate). J Nat Prod. 2004;67(12):2096–2098
- Yamasaki M, Kitagawa T, Koyanagi N, et al. Dietary effect of pomegranate seed oil on immunefunction and lipid metabolismin mice. Nutrition. 2006;22:54–59
- Chen Q, Jones TW, Brown PC, Stevens JL. The mechanism of cysteine conjugate cytotoxicity in renal epithelial cells. Covalent binding leads to thiol depletion and lipid peroxidation. J Biol Chem. 1990;265:21603–21611
- Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Ann Rev Biochem. 1985;54:305–329
- Ramanathan L, Das NP. Inhibitory effects of some natural products on metal-induced lipid oxidation in cooked fish. Biol Trace Elem Res. 1992;34(1):35–44
- Boussetta T, Raad H, Letteron P, et al. Punicic acid a conjugated linolenic acid inhibits TNFα-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS One. 2009;4(7):e6458
- Josh D, Mittal DK, Shukla S, Srivastav AK, Srivastav SK. N-acetyl cysteine and selenium protects mercuric chloride-induced oxidative stress and antioxidant defense system in liverand kidney of rats: A histopathological approach. J Trace Elem Med Bio. 2014;28(2):218–226
- Imaeda A, Kaneko T, Aoki T, et al. Antioxidative effects of fluvastatin and its metabolites against DNA damage in streptozotocin-treated mice. Food Chem Toxicol. 2002;40:1415–1422
- Saha SS, Dasgupata P, Sengupata S, Ghosh M. Synergistic effect of conjugated linolenic acid isomers against induced oxidative stress, inflammation and erythrocyte membrane disintegrity in rat model. Biochim Biophys Acta. 2012;1820(12):1951–70

