Abstract
Background: Stem cell therapy (SCT) is used for immunosuppression minimization in renal transplantation (RT). We carried out a prospective study to evaluate the benefits of co-infusion of donor adipose-derived mesenchymal stem cells (AD-MSC) + hematopoietic stem cells (HSC) in living donor RT (LDRT) under non-myeloablative conditioning. Methods: In a demographically balanced three-armed LDRT trial with 95 patients in each arm, group-1 received portal co-infusion of AD-MSC + HSC, group-2 received HSC and group-3 received no SCT. Lymphoid irradiation and anti-thyroglobulin were used for conditioning. Results: SCT was safe. At 1 and 5 years post-transplant, patient survival was 100% and 94.7% in group-1, 100% and 95.7% in group-2, and 94.7% and 84% in group-3, death-censored graft survival was 100% and 94.6% in group-1, 100% and 91.3% in group-2, and 98.9% and 94.4% in group-3 with mean serum creatinine (mg/dL) of 1.38 and 1.39 in group-1, 1.48 and 1.51 in group-2, and 1.29 and 1.42 and in group-3. Rejection episodes and immunosuppression requirement were lesser in SCT groups versus controls with best results noted in group-1. Conclusion: Coinfusion of donor AD-MSC +HSC in portal circulation pre-transplant under non-myeloablative conditioning is safe and effective for immunosuppression minimization in LDRT.
Introduction
Renal transplantation (RT) is a well-accepted therapy for end-stage renal disease (ESRD). However to prevent rejection of the grafted organ, patients need to be maintained on life-long immunosuppression which exposes them to high risk of infections, metabolic disorders and malignancy. The only solution is safe and effective reduction of immunosuppression while maintaining stable graft function. Options of powerful immunomodulators and stem cell therapy (SCT) are being explored to achieve transplantation with minimal/no immunosuppression.
We have been using SCT to achieve safe and effective minimization of immunosuppression in living donor renal transplantation (LDRT).Citation1 Mesenchymal stem cells (MSC) are believed to be powerful immunomodulators. We have been using hematopoietic stem cells (HSC) to induce tolerance with limited success.Citation2 We started using combined adipose tissue-derived MSC (AD-MSC) and HSC under non-myeloablative conditioning in LDRT in 2007. Early results showed that combined SCT was more effective than HSC alone in achieving improved survival and minimization of rejection episodes with minimization of immunosuppression.Citation3 We carried out the present study to compare the effect of portal co-infusion of AD-MSC with HSC in achieving minimization of immunosuppression while maintaining stable graft function in LDRT over a follow-up of more than 5 years and explored the reasons for our success.
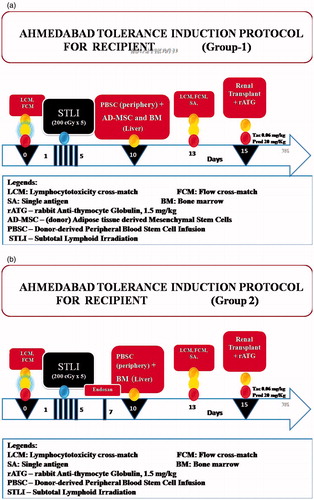
Study design
This was a prospective open-labeled un-blinded three-armed randomized clinical trial of LDRT performed between 2006 and 2010 with the use of our tolerance induction protocol (TIP) using donor AD-MSC and HSC in group-1, HSC in group-2, and group-3 of “controls” were not subjected to SCT. The patients were recruited into the protocols by explaining in detail all the procedures, risks, and possible benefits to each prospective allograft recipient and obtaining his/her written informed consent. Rabbit anti-thymocyte globulin (r-ATG), 1.5 mg/kg BW and total lymphoid irradiation (TLI) were given as induction therapy for groups-1 and 2, group-2 received cyclophosphamide in addition (). Group-3 received r-ATG alone for induction. TIP and informed patient/donor consent forms were approved by Institutional Review Board. RT was performed after favorable immune response noted by lymphocyte cross-match (LCM) and flow-cross-match (FCM). Maintenance immunosuppression in groups-1 and -2 consisted of Tacrolimus, 0.06 mg/kg BW and prednisone, 10 mg/d. Group-3 received mycophenolate sodium, 720 mg twice a day in addition.
Steps of protocol
Donor protocol for group-1
After baseline testing for HLA, LCM and FCM, donors were administered granulocyte colony-stimulating factor, 7.5 mg/kg BW subcutaneously twice a day on d 1, 2, 8 and 9. Adipose tissue resection was carried from anterior abdominal wall near umbilicus under local anaesthesia on day 1. On third day, for generation of HSC, 100 ml bone marrow (BM) was aspirated under local anesthesia and sedation under aseptic and anti-septic precautions. On the 10th day, donors were subjected to leucopheresis for procuring peripheral blood stem cells (PBSC) (Gambro China, version-7). Stitches of fat resection were removed on day 11. Donor nephrectomy for RT was performed on day 15 if immunologically favorable.
Recipient protocol for group-1 ()
After baseline testing for HLA, LCM and FCM, recipients were subjected to TLI (200 centiGray × 5 d) from day 1 to 5. On day 10, they were administered PBSC in periphery and ADMSC+BM-derived HSC in portal infusion. LCM, FCM were carried out on day 13. RT was performed on day 15 with favorable immune response.
The tests were considered favorable with FCM for T-cell match <50 and B-cell match <100 median channel shift [MCS], and AHG <20%. During transplantation, r-ATG, 1.5 mg/kg BW was administered. Methylprednisone, 500 mg IV was given on day 14, 15 and 16 (i.e., day “−1”, day “0” and day “+1” of transplantation), tapered to 250 mg on day 17 (i.e., 2nd postoperative day) and 125 mg on day 18 (i.e., 3rd postoperative day). Patients were closely supervised for all side effects due to SCT and conditioning and were managed as per standard guidelines.
Donor protocol for group-2 was similar to that of group-1 except that no adipose tissue resection was carried out.
Recipient protocol for group-2 ()
This was similar to that of group-1 except that patients were not administered AD-MSC. They were instead administered cyclophosphamide, 20 mg/kg BW on day 7.
HLA typing and cross-matching by LCM
HLA typing and LCM were performed using conventional serological techniques defined by American Society of Histocompatibility and Immunogenetics (ASHI) (one- Lambda pre-dot trays were used for HLA-A, B, DR typing), using auto cross-match, di-thiothretol (DTT) and standard cytotoxicity methods with mixed-cell population. LCM was performed as per ASHI guidelines with LCM, AHG and DTT.
Flow cytometry procedure for T and B-cell cross-match (FCM)
T and B lymphocytes were separately utilized for cross-matching. FCM was performed after enzyme (pronase) treatment to remove non-specific binding proteins. Fluorescent isothiocyanate-(FITC) conjugated anti-CD3 (perCP) and anti-CD 19 [phycoerythrin (PE)] monoclonal antibodies were used for labeling T and B-cells, respectively. Analysis was carried out by gating 5000 to 10,000 lymphocytes.
Stem cell generation
HSC
BM was transferred in to Dulbecco’s Modified Eagle’s medium (MEM) with added antibiotics and then immediately shifted to one with high glucose, essential amino acids, albumin, growth factors, and antibiotics. The medium was replenished every other day for 8–10 d, and supernatant was removed on day 10. Cultured marrow was mixed with AD-MSC after testing viability, sterility, staining, and content of BM and AD-MSC for group-1. For group-2, CBM alone was infused in recipients’ portal circulation.
AD-MSC
The adipose tissue was collected in medium containing α-MEM, 20% human albumin, and antibiotics and after mincing, transferred to a medium containing collagenase type 1 for digestion at 37 °C for 1 h on a locally designed shaker of Petri dishes (35–40 RPM). The contents of the medium were processed after centrifugation at 780 RPM for 8 min. Supernatants and pellets were separately cultured in medium of the same composition in 100-cm2 and 25-cm2 dishes (Sarsted), respectively, at 37 °C with 5% CO2 for 8–10 d. The medium was replenished every other day and harvested by trypsinization on 10th day. The collected cells were subjected to flow cytometric analysis. CD45 (perCP) negative, CD90 (PE) and CD73 (FITC) positive events were counted before the cells were mixed with cultured BM for portal infusion.
Procedure for cell count, viability and sterility checking
Cell count was performed in automated chamber (Coulter LH 750 analyzer) and a drop was preliminarily seen under microscope for contamination by bacteria–fungal elements. Viability of cells was checked using supravital staining technique in which 10 µL of cell aliquot was added to 10 µL trypan blue (supravital stain), mixed well, loaded on modified Neubaur’s chamber and subjected to light microscopy under 10× and 40× magnification. Cells with halo and no blue color, intact cell membranes were considered live and cells that picked up blue color stain due to ruptured membranes were counted dead. Live:dead cell ratio was prepared and more than 95% viability was considered acceptable.
A part of cell aliquot of 1 mL after viability testing was subjected to culture sensitivity in Paed plus bottles and placed in Bactec 6050. Sounding of a bip with red light alarm indicated growth of pathogenic bacteria and no signal till 4 d was considered as sterile. Samples were discarded if there was suspicious growth and/or viability was <95%.
SC were analyzed using FACScan (Becton Dickinson (BD) Biosciences, San Diego, CA). CD34+/CD45+ using CD34mAb (FITC-conjugated) and CD45mAb (PE-conjugated) (BD Biosciences) counting 50,000 events. For H-AD-MSC, CD45−/90+/CD73+, CD73mAb (PE-conjugated), CD90mAb (FITC-conjugated) and CD45mAb (PerCP-conjugated) were used and 20,000 events were counted.
Measurement of Trigs (CD127low/−/CD4+/CD25high) was performed from peripheral blood of patients of all groups using CD127 mAb (PerCP-Cy), CD4 mAb (PE), and CD25 mAb (FITC) according to manufacturer’s protocol using FACScan.
Portal infusion of stem cells
Under general anesthesia, a midline incision of approximately 3–5-cm length was made above the umbilicus by laparotomy, omental vein was identified and cannulated with 20 gauze intracath. Stem cell bag was connected and they were infused directly without using any filters, at the rate of 6–8 mL/min. After infusion, omental vein was ligated with silk and hemostasis was checked. The wound was closed with Vicryl 2/0, and subcuticular stitches were taken using 3/0 Monocryl.
Patients
Each group consisted of 95 patients. Demographics are mentioned in . The mean patient age was 31.3 years in group-1, 31.6 years in group-2 and 35 years in group-3, and males were predominant in all groups. The commonest original disease leading to ESRD was chronic glomerulonephritis followed by chronic tubulointerstitial nephritis and diabetic nephropathy followed by other diseases in all groups. The mean donor age (in years) was 47 in group-1, 48.4 in group-2 and 46.4 in group-3, with women as predominant donors in all groups. Donors were mainly parents followed by spouses, siblings and others in all groups. Donor–recipient match was 2.7 in groups-1 and -3, and 2.6 in group-2.
Table 1. Patient–donor demographics of the three groups subjected to renal transplantation.
Table 2. Follow-up of the three groups subjected to renal transplantation.
The mean total HSC infused were 8.85 × 108 nucleated cells/kg BW (recipient) in group-1 and 10.42 × 108 cells/kg BW in group-2. Mean CD34+ (n × 106 cells/kg BW) were 1.23 in group-1 and 1.8 in group-2. The mean CD45−/CD90+/CD73+ constituted 4.6 ± 2.5 × 104 cells/kg BW.
Post-transplant monitoring for all groups was done for complete blood counts and serum creatinine (SCr) at weekly intervals for the first month, fortnightly for 2nd month, monthly for the next 4 months, and then every 3 months. Immunosuppression minimization in groups-1 and -2 began with Tacrolimus replaced by mycophenolate/Sirolimus 1-year post-transplant. Tacrolimus levels were measured at weekly intervals for the first 2 months post-transplant, fortnightly for the next 2 months, and monthly thereafter (Siemens RxL Max, according to manufacturer’s protocol) maintaining trough levels 4–7 ng/mL. Sirolimus levels were also maintained at same levels as tacrolimus and were measured every month. Mycophenolate sodium level measurement was not performed. Graft biopsy was performed for dysfunction in all groups and managed as per standard guidelines. Protocol biopsies were performed in a subset of patients with stable graft function who gave their consent. Donor-specific antibodies (DSA) were measured in subset of patients in event of graft dysfunction and values > 2000 mean fluorescence intensity (MFI) was considered significant. Tregs were measured randomly in a subset of patients of all groups.
Statistical analysis
All statistical analysis was performed using the statistical program statistical package for the social sciences (SPSS version 12.0, Bengaluru, Karnataka, India). Data are expressed as mean ± SD for continuous variables and number (%) for categorical variables. Continuous variables were compared using analysis of variance (ANOVA). Chi-square tests of Fisher exact test were used to assess the effect of change in differences in categorical variables. Survivals were examined using Kaplan–Meier analysis and compared using the log-rank test for survival analysis between two groups. p < 0.05 was considered to be statistically significant.
Results
No side effects in the form of infections or graft-versus-host disease was observed after SCT/conditioning in any patient. Results are mentioned in Table 2.
At 1, 3, 5 and 7 years, the patient survival was 100%, 100%, 94.7% (not appeared at 7 years) in group-1, 100%, 97.9%, 95.7% and 92.5% in group-2, and 94.7%, 90.5%, 84% and 78.4% in group-3 (). Death-censored graft survival for the same time period was 100%, 98.9%, 94.6% (not appeared at 7 years) in group-1, 100%, 96.8%, 91.3% and 86.7% in group-2, and 98.9%, 94.4%, 94.4% and 94.4% in group-3 ().
Figure 2. (a) Kaplan–Meier curve for patient survival of the three groups. (b) Kaplan–Meier curve for death-censored graft survival of the three groups.
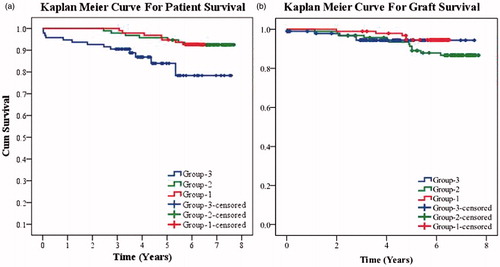
We lost 13 patients of the 95 in group-3, 9 of these 13 were lost due to infections. These patients were on triple immunosuppression exposing them to infections unlike the groups -1 and -2 where the patients were not immunocompromised. We lost one patient in group-2 due to infection, four patients to cardiovascular events and two were lost to cerebrovascular events. In group-3, two patients were lost due to infections, four were lost due to cardiovascular events and one due to cerebrovascular event.
Graft function of group-1 measured in terms of mean SCr (mg/dL) at 1, 3 and 5 years, were 1.38, 1.35 and 1.39, respectively. At 1, 3, 5 and 7 years, mean SCr (mg/dL) of group-2 was 1.48, 1.54, 1.51 and 1.55; and of group-3 was 1.29, 1.31, 1.42 and 2.06, respectively. Thus, although SCr was better in group-3 initially, after 5 years the graft function deteriorated in this group. Groups-1 and -2 had stable graft function over a longer follow-up.
Acute rejection (AR) episodes with Banff scores ≤ ag1 at1 av1 ai2 and PTC score G0 were noted in 20% of group-1; 30.5% of group-2 and 8.4% in group-3 whereas AR episodes with Banff scores > ag1 at1 av1 ai2 and PTC score> G0 were noted in 15.8% of group-3. Groups-1 and -2 were spared from more intense AR episodes. Chronic rejection (CR) episodes with Banff scores ≤ cg1 ct1 cv1 ci2 and PTC score G0 were noted in 3.2% of group-1; 11.6% of group-2 and 1.1% of group-3 whereas CR episodes with Banff scores > cg1 ct1 cv1 ci2 and PTC score >G0 were noted in 8.4% of group-3. Groups-1 and -2 were spared from more intense CR episodes. The incidence of rejection episodes was less in group-1 versus group-2.
Among patients with functioning grafts at the time of the analysis in group-1, 3.6% patients are on zero immunosuppression, 13.3% are on prednisone (5–10 mg/d) monotherapy, 51.8% on two immunosuppressants (mycophenolate + prednisone) and 31.3% are on triple immunosuppression (prednisone, mycophenolate and Tacrolimus/Sirolimus). In group-2, 1.3% patients are on zero immunosuppression, 5.3% on prednisone monotherapy, 56.6% on two immunosuppressants and 36.8% are on triple immunosuppression (as mentioned above). In group-3, all 77 patients are on triple immunosuppression. Thus, SCT has helped in safe immunosuppression minimization.
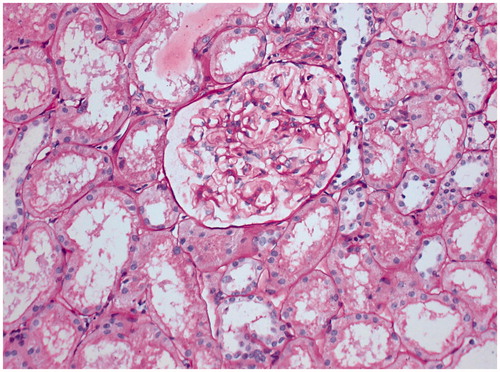
Unremarkable protocol biopsies were observed in all eight patients who gave their consent in group-1, three patients in group-2 and one patient in group-3. Representative biopsy is shown in .
Figure 3. Representative protocol biopsy of 59-years-old renal allograft recipient from group-1, who underwent transplantation with his sister’s kidney (HLA match – 1/6) on 31 October 2007. He is on maintenance immunosuppression of prednisone, 5 mg/d since 4 years. Periodic Schiff stain, ×200.
DSA were tested in 48 patients of group-1, 29 patients of group-2 and 5 patients of group-3. Of these, DSA class I was present in eight patients of group-1 and one patient of group-2. DSA class II was present in 14 patients of group-1, four patients of group-2 and two of group-3. Both DSA class I and II were present in six patients each in groups-1 and -2. Both class I and II were absent in 20 patients of group-1, 18 of group-2 and 3 of group-3. However, there was no correlation between rejection episodes and DSA. Mean Tregs were 3.02% in 72 patients of group-1, 2.5% in 57 patients of group-2 and 2.07% in 32 patients of group-3.
Discussion
The use of immunosuppression has led to improved survival of renal allografts at the cost of risk to patients in the form of opportunistic infections in the early stage and malignancies later on, apart from causing other cardiovascular and metabolic disorders. In spite of using potent adequate immunosuppression, rejections are not completely controlled. There is decreased incidence of AR especially with better HLA-matched grafts; however, there is still no answer to CR.
There are two well-accepted pathways by which a graft is rejected. In the direct pathway, the donor antigen is presented to the recipient T lymphocyte receptor by donor antigen presenting cell which is manifested as AR.Citation4 In the second route of indirect pathway, the graft-derived antigens (of MHC/non-MHC origin) are presented to the recipient immune system by the recipient’s own dendritic cells and is believed to be mainly responsible for CR.Citation5–8 Thus, if rejection needs to be arrested, both the pathways should be addressed.
Calcineurin inhibitor (CNI) have proved useful in arresting the direct pathway and thus bringing down the incidence of AR; however, CNI toxicity has led to search for better options for longer survival of patients and grafts. Various protocols for minimization of immunosuppression without adversely affecting the graft function have been tried. In a multi-center US–Europe trial of 98 patients, an immunosuppressive regimen consisting of induction therapy with the anti-IL-2 R antibody, Daclizumab and maintenance therapy with mycophenolate mofetil (MMF) and steroids there was an AR incidence of 53%.Citation9,Citation10 In another European study of 40 patients in which no induction therapy was used and where CNI was replaced by Sirolimus, the incidence of AR was 27.5%. These patients were also on MMF and prednisone.Citation11 Steroid minimization or withdrawal regimens have been tried with limited success by few; however, some centers have abandoned them due to excessive rejection rates.Citation12–14 Donor-specific transfusions had shown promising results in terms of inducing donor-hyporesponsiveness and reduction of rejection episodes.Citation15 The success was even attributed to deletion of rejecting immune repertoire.Citation16 This encouraged the use of concomitant donor BM infusion in solid organ transplantation. Reduction in both AR and CR was observed.Citation17–19 Further, combined approach of using stem cell infusion with TLI followed by weaning off immunosuppression at 1 year was also tried and showed acceptable outcomes.Citation20,Citation21 In a study, immunoregulatory macrophages called transplant acceptance-inducing cells (TAICs) were infused in five renal transplant patients pre-transplant for stepwise minimization of Tacrolimus in an immunosuppression regimen consisting of ATG, Tacrolimus and Prednisone.Citation22 Although there was no conclusive evidence of beneficial effect of pre-operative TAIC treatment, at least encouraging results in terms of safety of cell-based therapy were established.
In a study of 159 renal allograft recipients, divided in to three groups, autologous BM-derived MSC were infused at the time of anastomosis and 2 weeks later in group-1 with 53 patients, group-2 with 52 patients, and group-3 patients were treated as controls and were not given any stem cells.Citation23 Group-1 received standard CNI, group-2 received low-dose CNI and the control group received anti-IL-2 receptor antibody along with standard dose CNIs. The survival was similar in all groups, however the incidence of AR was lower in patients who received MSC versus controls. The incidence of AR was 7.5% in group-1, 7.7% in group-2 and 21.6% in group-3, noted at 6 months post-transplant. In addition, the rejections in groups 1 and 2 responded to steroids whereas four patients of control arm had steroid-resistant rejections. The incidence of opportunistic infections was also lower in patients who received MSC as compared to controls.
The estimated renal functions were superior in patients who received MSC as compared to controls. This study showed encouraging results with the use of MSC as observed in our study. In fact this study shows short-term advantages, whereas our study of 5 years has shown overwhelmingly good results in the form of stable graft function with minimum infections, minimum and less severe AR and CR, thus paving the way for transplant tolerance.
The present study establishes that cell-based therapy is safe when used preoperatively if the cells are infused in portal circulation. While comparing the survival status and rejection episodes, patients subjected to SCT have shown significant improvement. The benefits cannot be attributed to conditioning regimens alone, since all the studies where powerful conditioning regimens have been used, have shown increased mortality and morbidity due to infections and other side effects resulting from immune incompetence of recipients.Citation24–27 Although SCr appears significantly better in control arm of our study for the first 3 years, these effects are counter-balanced by 5 years post-transplant in the form of significantly decreased survival and raised SCr in this group of patients in spite of using optimum triple immunosuppression.
If we compare the results of group-1 with group-2 where combination of AD-MSC and HSC was used in group-1 versus HSC alone in group-2, both short-term and long-term patient survivals are good. However, graft survival was significantly better in group-1 where AD-MSC was used. Mean SCr, rejection episodes and immunosuppression requirement was also less in the former. Protocol biopsies could not be performed in more patients due to reluctance of patients. However, attempts are still being made to convince the patients to give consent for biopsy. Group-2 was administered more HSC than group-1, this clearly supports our notion that MSC have beneficial effect over HSC.
If the stem cells are injected in periphery they get rejected or even get trapped in lungs causing life-threatening situation.Citation28 When infused in portal circulation, stem cells stay in hepatic microcirculation leading to a chain of tolerogenic events for induction and maintenance of tolerance. It believed to be related to switching from Th1 to Th2 cells with consequent changes in the cytokine milieu favoring levels of IL4 and IL10 thereby preventing rejection.Citation29,Citation30 PBSC being rich in Tregs was infused in periphery. MSCs suppress the T lymphocyte proliferation induced by alloantigens, mitogens and anti-CD3 and anti-CD28 antibodies in vitro, in humans, baboons and mice.Citation31–35 MSCs have a similar effect on memory and naive T cells as well as CD4+ and CD8+ T cells and this suppressive effect does not require major histocompatibility complex (MHC) restriction.Citation36,Citation37 MSCs induce B-cell suppression by physical contact and release of certain soluble factors.Citation38 MSCs isolated from human adipose tissue are more potent immunomodulators for the differentiation of human dendritic cells than MSCs derived from the BM.Citation39 MSCs also can induce kidney allograft tolerance by inducing the generation of CD4+CD25+FoxP3+ T-regulatory cells in vivo. Additionally, MSCs have been reported to induce the formation of CD8+ T-regulatory cells that are responsible for the inhibition of allogeneic lymphocyte proliferation.Citation40 These evidences and our study confirm the hypothesis that MSC are potent immunomodulators and have helped in induction of Prope’ tolerance in RT.Citation41 In addition immunosuppression minimization not only reduces the chances of infections, malignancy, cardiovascular and other metabolic risk factors, but also has additional benefits of reducing the financial burden of a family especially in countries where medical allowances/care are not available to solid organ transplant recipients. This fact is well established in our study. The cost of maintenance immunosuppression in control patients who are not offered this stem cell protocol is approximately US $ 250 to 300 per month. In addition whenever the patients incur infection, the cost is approximately US $ 500. As compared to this cost, patients who undergo TIP using stem cells, incur a monthly cost of < US $ 25 approximately. Thus, patients have the advantage of saving about 10 times in addition to returning to mainstream of life after few months of stable graft function.
Shortcomings of the present study
In the present study, we did not have expertise of stem cell infusion by catheterization through femoral/radial vessels that is now well established in our set-up. Hence, our patients had to undergo general anesthesia. We have not been able to monitor DSAs of all patients due to financial constraints. Our recent studies of monitoring with Tregs have shown interesting correlation that Tregs between 3 and 3.5% correlate with stable graft function (unpublished data). However, we have not been able to monitor Tregs in these patients due to unavailability of kits as well as financial constraints. We did not perform chimerism studies in all patients of the present study since we did not find any correlation between chimerism and tolerance induction.
Conclusion
Co-infusion of donor adipose tissue derived mesenchymal and hematopoietic stem cells in portal circulation pre-transplant under non-myeloablative conditioning is safe and effective strategy for minimization of immunosuppression in LDRT. AD-MSC is likely to help us reach the destination of immune tolerance with additional support of Tregs.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article.
Acknowledgements
We appreciate the Transplant surgeons Prof. Pranjal Modi and J. Saiyed Rizvi for performing transplant surgeries, Dr. V. Shankar for giving TLI, Ms. Varsha Trivedi and her team for performing HLA typing and lymphocyte cross-matching, Professors Rashmi Patel and Kamal Kanodia and technologists for supporting with lab work including histopathology, Mrs. Priyadarshini Shah, Transplant Coordinator, for taking care of our patients and providing the data, and Mrs. Shobhana Sengunthar for performing statistical analysis.
References
- Vanikar AV, Goplani KR, Feroz A, et al. Operational tolerance in living-related renal transplantation: A single-center experience. Transplant Proc. 2011;43(5):1551–1558
- Trivedi HL, Vanikar AV, Modi PR, et al. In pursuit of the ultimate: The initial Ahmedabad journey toward transplantation tolerance. Transplant Proc. 2007;39(3):653–657
- Vanikar AV, Trivedi HL, Feroz A, et al. Effect of co-transplantation of mesenchymal stem cells and hematopoietic stem cells as compared to hematopoietic stem cell transplantation alone in renal transplantation to achieve donor hypo-responsiveness. Int Urol Nephrol. 2011;43(1):225–232
- Nakajima H, Leonard WJ. Impaired peripheral deletion of activated T cells in mice lacking the common cytokine receptor gamma chain. J Immunol. 1997;159:4737–4744
- Fangmann J, Dalchau AR, Fabre JW. Rejection of skin allografts by indirect allorecognition of donor class I major histocompatibility complex peptides. J Exp Med. 1992;175:1521–1529
- Sherwood RA, Brent L, Rayfield LS. Presentation alloantigens by host cells. Eur J Immunol. 1986;16:569–574
- Auchincloss H Jr. In search of the elusive Holy Grail: The mechanisms and prospects for achieving clinical transplantation tolerance. Am J Transplant. 2001;1:6–12
- Shirwan H. Chronic allograft rejection: Do the Th 2 cells preferentially induced by indirect alloantigen recognition play a dominant role? Transplantation. 1999;68:715–726
- Vincenti F. Immunosuppression minimization: Current and future trends in transplant immunosuppression. J Am Soc Nephrol. 2003;14:1940–1948
- Vincenti F, Ramos E, Brattstrom C, et al. Multicenter trial exploring calcineurin inhibitors avoidance in renal transplantation. Transplantation. 2001;71:1282–1287
- Kreis H, Cisterne J-M, Land W, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation. 2000;69:1252–1260
- Ahsan N, Hricik D, Matas A, et al. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil — A prospective randomized study. Steroid Withdrawal Study Group. Transplantation. 1999;68:1865–1874
- Vanrenterghem Y, Lebranchu Y, Hene R, et al. Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation. 2000;70:1352–1359
- Squifflet J-P, Vanrenterghem Y, van Hooff JP, et al. Safe withdrawal of corticosteroids or mycophenolate mofetil: Results of a large, prospective, multicenter, randomized study. Transplant Proc. 2002;34:1584–1586
- Salvatierra O, Melzer J, Potter D, et al. A seven-year experience with donor-specific blood transfusions. Results and considerations for maximum efficacy. Transplantation. 1985;40:654–659
- Terasaki PI. The beneficial transfusion effect on kidney graft survival attributed to clonal deletion. Transplantation. 1984;37:119–125
- Kirk AD, Mannon RB, Swanson SJ, Hale DA. Strategies for minimizing immunosuppression in kidney transplantation. Transplant Int. 2005;18:2–14
- Ricordi C, Karatzas T, Nery J, et al. High-dose bone marrow infusions to enhance allograft survival. The effect of timing. Transplantation. 1997;63(1):7–11
- Ciancio G, Miller J, Garcia-Morales RO, et al. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation. 2001;71:827–835
- Buhler LH, Spitzer TR, Sykes M, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405–1409
- Millan MT, Shizuru JA, Hoffmann P, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73:1386–1391
- Hutchinson JA, Brem-Exner BG, Riquelme P, et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transplant Int. 2008;21:742–754
- Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants – A randomized controlled trial. J Am Med Assoc. 2012;307(11):1169–1177
- Kirk AD, Mannon RB, Kleiner DE, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80:1051–1059
- Szczech LA, Berlin JA, Feldman HI. Anti-lymphocyte antibody induction therapy study group. The effect of anti-lymphocyte induction therapy on renal allograft survival. A meta-analysis of individual patient-level data. Ann Intern Med. 1998;128:817–826
- Peddi VR, Bryant M, Roy-Chaudhury P, et al. Safety, efficacy, and cost analysis of Thymoglobulin induction therapy with intermittent dosing based on CD3+ lymphocyte counts in kidney and kidney-pancreas transplant recipients. Transplantation. 2002;73:1514–1518
- Meier-Kriesche HU, Arndorfer JA, Kaplan B. Association of antibody induction with short- and long-term cause specific mortality in renal transplant recipients. J Am Soc Nephrol. 2002;13:769–772
- Jae Woo Jung, Minsuk Kwon, Jae Chol Choi, et al. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J. 2013;54(5):1293–1296
- Ikehara S. New strategies for allogeneic bone marrow transplantation and organ allografts. Acta Hematol. 1999;101:68–77
- Gorczynski RM, Chen Z, Kai Y, Lei J. Evidence for persistent expression of OX2 as a necessary component of prolonged renal allograft survival following portal vein immunization. Clin Immunol. 2000;97(1):69–78
- Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843
- Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75:389–397
- Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729
- Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729
- Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B- cell functions. Blood. 2006;107:367–372
- Ivanova-Todorova E, Bochev I, Mourdjeva M, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42
- Ge W, Jiang J, Arp J, et al. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312–1320
- Calne RY, Friend PJ, Moffatt S, et al. Prope tolerance, perioperative Campath 1H, and low dose cyclosporine monotherapy in renal allograft recipients (letter). Lancet. 1998;351:1701–1702