Abstract
Introduction: As intensive care units (ICU) have improved, presence of multiple-organ dysfunctions in majority of patients with acute renal failure (ARF) has become clearer. To facilitate multi-organ support, continuous renal replacement therapy (CRRT) techniques have been developed. This study is the one that reports the experience on children including newborns receiving CRRT monitored in ICU. Materials and Methods: The study was performed retrospectively in children who had Continuous Veno- Venous Hemodiafiltration (CVVHDF) as a CRRT modality in ICU. Clinical data, primary cause, consultation time, duration and initiation time of CVVHDF were recorded. Patients were classified as cardiac and non-cardiac in respect to primary dysfunction. Stage of renal failure was evaluated according to pRIFLE criteria. Outcome was identified as primary and secondary. Primary outcome was accepted as the composite correction of uremia and metabolic parameters, and regression of fluid overload, while secondary outcomes were assessed as improvement of hemodynamic instability and survival. Results: A total of 36 patients’ files were scanned. There were 10 cases in cardiac group and 26 cases in non-cardiac group. There were statistically better differences between primary and secondary outcome rates of cardiac cases. Although there was no difference between cardiac and non-cardiac cases in terms of primary outcome, secondary outcome was statistically significant. Timing of consultation and CVVHDF was not found to have an effect on the outcome. Conclusion: Our results indicated that CVVHDF treatment was successful even in cardiac patients with high mortality and in patients at their later stage of ARF.
Introduction
The prevalence of acute renal failure (ARF) in the intensive care units (ICU) has been reported as 10% and 70% of the cases diagnosed with ARF require renal replacement therapy (RRT).Citation1–4 Acute renal impairment-dependent mortality rate was above 50% in cases who received RRT. Since continuous renal replacement therapy (CCRT) modalities provide sustainable blood clarification and they are adequate to systematic fluid removal in children who are in a critical condition in the ICU, CRRT modalities have been among the first and the most common modalities compared to peritoneal dialysis (PD) or intermittent hemodialysis (IHD).Citation5–9 It has been observed that ARF occurs particularly aftermath of the cardiac operations and CRRT is initiated at the rate of 1–17% in children of this group.Citation10 Despite the fact that lesser body surface area and body weights of the pediatric patients lead various kinds of problems in performing CRRT, outcome studies appearing in the pediatric nephrology literature confirm that clinicians’ CRRT-based treatment choices rather than IHD and PD.Citation11,Citation12
Our purpose in this study is to reveal the factors that affect the outcome and treatment responses of the cases to which we performed Continous Veno-Venous Hemodiafiltration.
Materials and methods
Clinical data of 36 cases initiated on CVVHDF between the dates of June 2009 and June 2013 by Ege University – Pediatric Nephrology Clinic were analyzed, retrospectively. Cases were admitted to pediatric and cardiovascular ICU, and they were presented to us due to the diagnosis of ARF and/or multiple-organ dysfunction.
Cases who received IHD or PD due to acute renal impairment and extracorporeal membrane oxygenation (ECMO) or extracorporeal life support were excluded from the study. Demographic data, body weight, diagnosis, CVVHDF indications, treatment duration extracted from the patient files were recorded by being reviewed.
CVVHDF indications were classified as non-renal indications in metabolic diseases (severe lactic acidosis) and treatment-resistant metabolic changes. The stage (R/I/F) of the cases receiving CVVHDF due to renal causes was determined in regard to pRIFLE criteria during the consultation.
Information such as, timing of consultation request, at which hour of the patients’ admission to the ICU they were consulted with nephrology, and how many hours later CVVHDF treatment was initiated after the consultation were recorded as the initiation time of CVVHDF. In addition, cardiac assessments, ventilator requirement, time spent in ICU, whether any complications develop due to CVVHDF treatment, and information of SOFA (Sequential Organ Failure Assessment) score were recorded by drawing from patients’ files. SOFA scores of the patients were calculated in respect to the clinical and laboratory values of CVVHDF performance on the first day, all systems were graded as between 0 and 4 by evaluating the functions of respiratory, liver, cardiovascular, renal, and central nervous system, and clotting.Citation13
Acute renal impairment and its classification were performed in respect to the pRIFLE criteria and CVVHDF was initiated in the R or I stage depending on the patients’ clinical conditions.Citation14 Glomerular filtration rate (GFR) was calculated with Schwartz formula.Citation15 Volume excess was described, as overloaded fluid balance non-responsive to diuretic treatment even there was no anuria. In the course of initiation CVVHDF treatment, fluid overload percent was calculated according to the formula developed by Goldstein and his friends [fluid intake − output/body weight (kg) checked in the ICU × 100].Citation16 The infants younger than or equal to 30 d of age were considered as newborns on the day of CVVHDF initiation.
Vascular access was enabled with double-lumen IHD catheter (7–11 French, Medcomp, Harleys Ville, PA) selected in conformity with the age and body weight of the patient inserted to internal jugular vein or femoral vein. Prizma (Cobe-Gambro Healthcare, Lakewood, CO) CRRT device was used in all patients for the procedure. Dialysate and ultrafiltration flow rates were adjusted according to the diagnosis of the patients (15–20 mL/min/m2 of body surface area in their ARF, and 15–20 mL/min/m2 of body surface area in their multi-organ failure: MOF) hemodynamic parameters, and required volume load. Moreover, replacement fluid calculated as 30–35 mL per kilogram rose to 60 mL/kg in cases with sepsis and metabolic diseases. For the purpose of enabling cytokine absorption in the circulation, M10 (Cobe-Gambro Healthcare, Lakewood, CO) was used for the cases under 10 kg, acrylonitrile (AN69) hemodialyzer membrane (M60, Gambro, Lakewood, CO) was used for those who weighed between 10 and 25 kg, and PS-polysulfone hemodialyzer (HF1000, Gambro) was used for those who weighed more than 25 kg.
Low-dose molecular weight heparin was used as an anti-coagulant, and anti-coagulant dose adjustment was performed in regard to the aPTZ (active partial trombine time) values by following hemorrhagic diathesis of the patient at intervals of 4 h. Solution selection was made according to the levels of serum potassium (includes Multibic 0: potassium-free, Multibic 2: 2 mEq/L potassium, Multibic 4: 4 mEq/L potassium). All procedures were performed with motorization in the ICU, and follow-up of 1-h for vital result; biochemical parameters at intervals of 8 h (liver–renal function tests, ions), blood glucose once in 1–2 h, and close blood gas were performed.
Patients were divided into two groups as cardiac and non-cardiac according to the etiology of the patients’ current diseases. Outcome was evaluated as primary and secondary. Correction of acidosis and metabolic parameters, GFR’s returning to normal and detection of fluid overload regression were accepted as the primary outcome, while enabling hemodynamic instability and outcome of the patient were assessed as the secondary outcome. CVVHDF treatment was terminated in cases with sufficient urine output (at least 1 mL/kg/h), stabile renal functions test results, and in cases where intake–output fluid balance was ensured; in other words, when primary outcome was achieved. Patients were examined in four different groups in regard to their primary and secondary outcome conditions; group 1 was specified as having primary and secondary outcome, group 2 was described as having only primary outcome, group 3 was described as having only secondary outcome, and group 4 was described as having neither primary nor secondary outcomes.
All data were specified as mean ± SS. “z” test was used for the comparison of outcome rates, and “t” test was used for the comparison of means while performing the statistical analysis.
Results
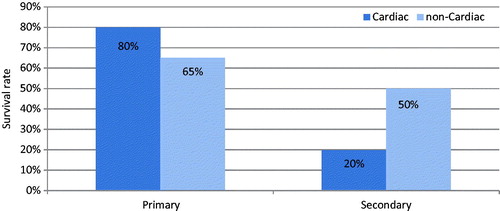
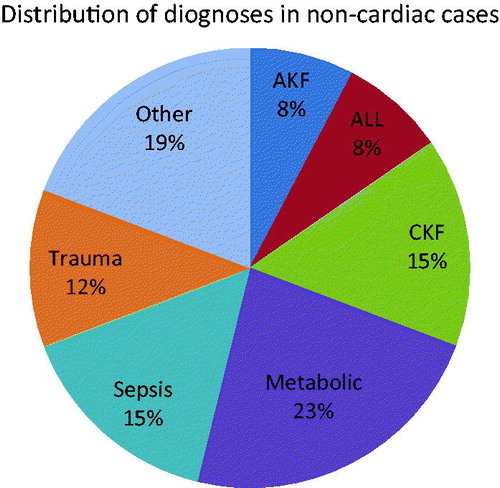
Thirty-six patients were included to this study as 18 males and 18 females. Patients’ mean age was 7.3 ± 2.16 years, and mean body weight was 23.22 ± 5.98 kg. It was determined that 27.7% of the patients were cardiac-caused, while 72.2% (n = 26) of the cases had non-cardiac symptoms with metabolic weight diseases (23%; ). Non-renal causes were consisted of seven cases among the indications of CVVHDF initiation (six cases had severe lactic acidosis, one case had treatment-resistant metabolic changes). Distribution made according to the pRIFLE criteria for the remaining 29 renal-dependent cases was 7/12/10, respectively (R/I/F). Renal causes leading initiation of CVVHDF treatment were acute renal impairment and/or volume load, sepsis, and MOF. Duration of CVVHDF treatment was so variable, the shortest lasted 1 h due to the development of cardiac arrest in a case diagnosed with MOF and the longest period was 315 h in a 10-year-old case in whom cardiac transplantation was performed, having MOF by reason of secondary hypoxia in extracorporeal circulation in the course of the transplantation. This case responded to CVVHDF treatment, however; became arrest while being performed aspiration due to obstruction in the endotracheal tube and did not respond to resuscitation. Eight cases of the 10 (80%) in the cardiac group were observed to have primary outcome, while only two cases (20%) were detected to have secondary outcome.
Figure 1. Distribution of diagnoses in non-cardiac cases. Note: AKF, acute kidney failure; CKF, chronic kidney failure; ALL, acute lymphoblastic leukemia.
Seventeen cases of the 26 (65%) in the non-cardiac group were observed to have primary outcome, while 13 cases (50%) had secondary outcome.
Primary outcome rates were not significantly different between the cases with cardiac and non-cardiac (z-test, z = −0.85), while there was a significant difference in secondary outcome rates (z-test, z = 1.6353).
While there was a significant difference in primary and secondary outcome rates of cardiac cases (z-test, z = 2.68), no significant difference could be detected in non-cardiac cases (z-test, z = 1.12; ).
When cases were examined in four different groups according to their primary and secondary outcome conditions, case numbers in these groups were 11/14/4/7, respectively.
Timing of consultation request within these groups, timing of CVVHDF initiation, and the connection between their timing of CVVHDF are shown in (). All comparisons of the four groups showed no statistically significant difference in terms of timing of consultation request and CVVHDF initiation. There was a significant difference detected between the group 2 and group 4 when comparing their duration of CVVHDF treatment (CVVHDF duration) ().
Figure 3. Consultation and CVVHDF time according to primary and secondary survival status. Notes: *Group 1: Primary survival – yes, secondary survival – yes. **Group 2: Primary survival – yes, secondary survival – no. ***Group 3: Primary survival – no, secondary survival –yes. ****Group 4: Primary survival – no, secondary survival – no.
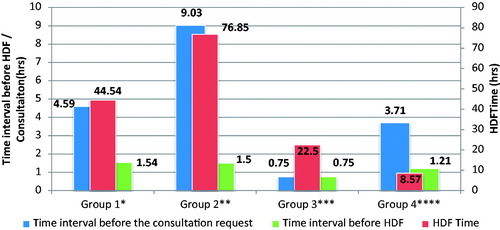
Table 1. Consultations.
Table 2. Timing of CVVHDF initiation.
Table 3. CVVHDF duration.
Discussion
In recent years, diseases requiring acute CRRT have been presented as the comorbidity of underlying another disease occurring particularly in children rather than the primary renal impairment. The most commonly reported causes of ARF are congenital cardiac diseases, bone marrow transplantation, nephrotoxins, and sepsis.Citation17 In our study, 5.5% of the patient groups had primary renal impairment. Moreover, again in conformity with the literature, impairment depending on congenital cardiac disease was the most common one among the CRRT indications, and metabolic diseases were considered as a second indication.Citation17
Impairment being the most common cause depending on congenital cardiac disease was associated with renal ischemia developed after cardiogenic shock in the literature.Citation6 Outcome rate in the literature was reported as between 34 and 52% in patients receiving CRRT. Likewise, 15 cases involved in our study of the 36 lived, and our outcome rate was determined as 42%.Citation5,Citation18–21 However, most of the cases have cardiac diseases, sepsis, and MOF, and require mechanic ventilator effect outcome rates.
Even correction of metabolic parameter was achieved with CRRT in particularly cardiac-caused patients; they could not be saved by reasons of insufficiency of cardiac functions and the low-ejection fraction. As a matter of fact, secondary outcome rates of non-cardiac cases were found to be statistically higher than cardiac cases. For this reason, we discussed the outcome as primary and secondary to assess the success of CVVHDF treatment performed as CRRT modality in our study. We evaluated the primary outcome as having a response to CVVHDF treatment, however; we also mentioned the presence of secondary outcome in surviving patients. In this regard, patients in group 1 and group 2 responded to CVVHDF treatment and the percentage of (69%) these patient groups was interpreted as the efficacy of CVVHDF. In the study performed by Mehta et al. morbidity rate was higher in patients who were performed CRRT, while no difference could be found in renal outcome. In a national-scale retrospective analysis that was performed by Bell et al. in Switzerland ICU, recovery rate of the patients’ renal functions was also detected to be higher.Citation22,Citation23
In a study performed by Proulx et al., it was stated that contrary to adult patients, pediatric patients had MOF in a shorter period of time in the course of the ICU and for this reason, could not be saved.Citation24 Therefore, managing early diagnosis also falls into the factors that affect the outcome process as well as CRRT modality preventing the indications of IHD or PD.
CRRT success has increased, thanks to Akın et al. for bringing forward the pRIFLE criteria in 2007 continuing the extensive use of these criteria day by day.Citation14 Goldstein et al.’s study, which was composed of 22 pediatric patients, observed that timing of CVVHDF initiation had no effect on the outcome.Citation5 Also, our study detected no statistically significant effect of consultation period of the patients admitted to ICU and the timing of CVVHDF initiation on the outcome rates. On the other hand, this inefficacy may arise from patients’ being performed a quick consultation, and patients’ receiving CRRT in the early period by being staged in respect to the pRIFLE criteria.
Significant difference was found in groups 2 and 4 when compared with their CVVHDF treatment durations (CVVHDF duration), however; these results are not quite reliable since the amount of data is limited. CVVHDF duration in the second group, where only primary outcome is present, is relatively longer compared to the other three groups, but this difference appears to be statistically significant only in the fourth group. CVVHDF modality preferred in patients with poor prognosis can be related to the high-morbidity rate (58%) due to the presence of hemodynamic instability, MOF, and additional diseases.
As a conclusion, our study indicated that CVVHDF treatment had efficacy in primary outcome of the cardiac patients with high-morbidity rate, even it did not have the significant effect on secondary outcome of the pediatric patients in the ICU.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article.
References
- Tantalean JA, Leon RJ, Santos AA, Sanchez E. Multiple organ dysfunction syndrome in children. Pediatr Crit Care Med. 2003;4(2):181–185
- Williams DM, Sreedhar SS, Mickell JJ, Chan JC. Acute kidney failure: A pediatric experience over 20 years. Arch Pediatr Adolesc Med. 2002;156(9):893–900
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294(7):813–818
- Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008;19(6):1233–1238
- Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–1312
- Lopez-Herce J, Santiago MJ, Solana MJ, et al. Clinical course of children requiring prolonged continuous renal replacement therapy. Pediatr Nephrol. 2010;25(3):523–528
- Strazdins V, Watson AR, Harvey B. European Pediatric Peritoneal Sialysis Working G. Renal replacement therapy for acute renal failure in children: European guidelines. Pediatr Nephrol. 2004;19(2):199–207
- Walters S, Porter C, Brophy PD. Dialysis and pediatric acute kidney injury: Choice of renal support modality. Pediatr Nephrol. 2009;24(1):37–48
- Pasko DA, Churchwell MD, Btaiche IF, Jain JC, Mueller BA. Renal replacement therapy kinetics study G. Continuous venovenous hemodiafiltration trace element clearance in pediatric patients: A case series. Pediatr Nephrol. 2009;24(4):807–813
- Miyamoto T, Yoshimoto A, Tatsu K, Ikeda K, Ishii Y, Kobayashi T. Zero mortality of continuous veno-venous hemodiafiltration with PMMA hemofilter after pediatric cardiac surgery. Ann Thorac Cardiovasc Surg. 2011;17(4):352–355
- Shiga H, Kikuchi Y, Hattori N, Hirasawa H. Special considerations in continuous hemodiafiltration with critically ill pediatric patients. Contribut Nephrol. 2010;166:158–166
- Odetola FO, Clark SJ, Freed GL, Bratton SL, Davis MM. A national survey of pediatric critical care resources in the United States. Pediatrics. 2005;115(4):e382–e86
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710
- Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035
- Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(3):571–590
- Goldstein SL. Overview of pediatric renal replacement therapy in acute kidney injury. Semin Dial. 2009;22(2):180–184
- Soysal DD, Karabocuoglu M, Citak A, Ucsel R, Uzel N, Nayir A. Metabolic disturbances following the use of inadequate solutions for hemofiltration in acute renal failure. Pediatr Nephrol. 2007;22(5):715–719
- Maxvold NJ, Smoyer WE, Gardner JJ, Bunchman TE. Management of acute renal failure in the pediatric patient: Hemofiltration versus hemodialysis. Am J Kidney Dis. 1997;30(5 Suppl 4):S84–S88
- Smoyer WE, McAdams C, Kaplan BS, Sherbotie JR. Determinants of survival in pediatric continuous hemofiltration. J Am Soc Nephrol. 1995;6(5):1401–1409
- Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–658
- Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: A retrospective analysis. Crit Care Med. 2004;32(8):1771–1776
- Mehta RL, McDonald B, Gabbai FB, et al. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60(3):1154–1163
- Bell M, Granath F, Schon S, Ekbom A, Martling CR. Continuous renal replacement therapy is associated with less chronic renal failure than intermittent hemodialysis after acute renal failure. Intensive Care Med. 2007;33(5):773–780
- Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22(6):1025–1031