Abstract
Objectives: To evaluate whether the outcomes of renal grafts from living related donors older than 60 years are acceptable, in terms of renal function and patient/graft survival. Material and methods: One hundred and forty-seven patients who received kidneys from donor age ≥60 years constituted the study group (group 1). The control group (group 2) consisted of 1310 patients who received renal transplants from donor age <60 years. Outcome measures included graft, patient survival, acute rejection rate and serum creatinine (SCr) in patients/donors. Graft and patient survivals were compared using the Kaplan–Meier method. Results: The mean age of donors was 62.7 ± 3.39 years in group 1 and 43.45 ± 9.65 years in group 2. Patient survival at 1, 3 and 5 years was 95.7%, 89.4% and 82.6% in group 1 and 93.8%, 89.1% and 83.1% in group 2 (p = 0.785), respectively. Death-censored graft survival at 1, 3 and 5 years was 98.5%, 94.8% and 94.8% in group 1 and 96.1%, 92.9% and 89% in group 2 (p = 0.166), respectively. Biopsy-proven acute rejections were 21% and 16.8% (p = 0.206) and chronic rejections 5% and 3.4% in group 1 and 2, respectively (p = 0.542). Recipient SCr (mg/dL) was 1.8 ± 0.31 in group 1 and 1.58 ± 0.37 in group 2. The donor SCr levels at the last follow-up were 1 mg/dL and 0.9 mg/dL in group 1 and 2, respectively. Conclusions: Donor age did not affect patient and graft survival in the 5-year follow-up in our study. Age alone seems not to be an exclusion criterion to living kidney donation.
Introduction
The Indian chronic kidney disease (CKD) registry recently published its first report, which showed that of all the stage V CKD cases, a majority (61%) were not on any form of renal replacement therapy at the time of reporting, 32% on hemodialysis, 5% on peritoneal dialysis and 2% were being worked up for transplantation.Citation1 India has a renal transplantation (RTx) rate of 3.25 per million of population (PMP) per year, and the deceased donation rate is 0.08 PMP per year, which is grossly inadequate.Citation2
It is not always possible to find an ideal living donor (LD). Sometimes, the only available donor in a family has some benign disease or suffers suboptimal renal anatomy or physiology, or is too old. Older LDs are presumed to provide an inferior-quality kidney. However, the utilization of highly selective older LDs can be a safe, feasible and effective way for the treatment of patients with end-stage renal disease (ESRD). The disparity between donor kidney availability and demand has increased utilization of kidneys from older LDs.Citation3–9 Using grafts from older donors is increasing in Europe and the United States. Data scarcity of transplant outcome from older LDs prompted us to review our experience. In order to better understand the effect of donor age on LDRTx, we examined LDRTx outcomes in donor age ≥ 60 years (group 1) and donor age < 60 years (group 2).
Materials and methods
A total of 1457 LDRTx recipients on regular follow-up at our center from 2007 to 2012 were studied. This study was performed with the approval of our local hospital research ethics board.
The specific study objective was to determine LDRTx outcomes in two groups. We retrospectively collected data of LDs and recipients. Outcome measures included graft, patient survival and acute rejection rate. Donor kidneys were extensively assessed at the time of donation using imaging and laboratory tests; the results were satisfactory. Surgical risks for donors and recipients and potential compromising graft functions for recipients were fully informed. All donors in both the groups were followed up and serum creatinine (SCr) level was evaluated regularly. Tc99m-DTPA (diethylene triamine pentaacetic acid) renal scan was performed in all donors and all had glomerular filtration rate (GFR) of > 40 mL/min on either side. Body mass index, blood pressure, lipid profile, fasting blood glucose, postprandial blood glucose and oral glucose tolerance test were done in all donors and were normal before kidney donation.
All the patients received induction immunosuppressive therapy with methylprednisolone (500 mg intravenously for three days) and rabbit-antithymocyte globulin (1.5 mg/kg, single dose) in high immunologic risk group. Calcineurin inhibitor-based regimen was used for maintenance immunosuppression. Graft biopsy was performed in cases of acute graft dysfunction, diagnosed as per the modified Banff classification and treated according to standard guidelines.
Statistical analysis was performed using Statistical Package for the Social Sciences (version 12.0; SPSS Inc., Chicago, IL). Continuous variables were compared using Student T-test. Chi square test of Fisher exact test were used to assess the effect of change in differences in categorical variables. Survivals were examined using Kaplan–Meier analysis and compared using the log-rank test. p-Value < 0.05 was considered statistically significant.
Results
Recipient and donor characteristics in group 1 (donor age ≥ 60 years)
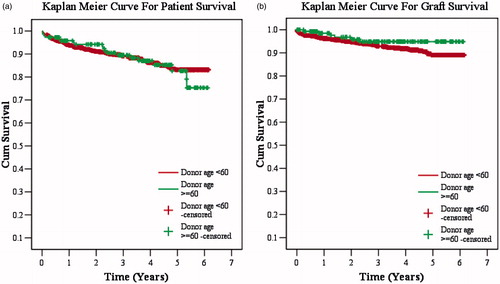
There were 147 patients in group 1. There were 123 males and 24 females, with a mean age of 36.58 ± 9.9 (range 11–73 median 35 years). Original diseases leading to ESRD were chronic glomerulonephritis (CGN) (n = 73), diabetic nephropathy (DN) (n = 14), hypertension (HTN) (n = 14), chronic interstitial nephritis (CIN) (n = 7), obstructive uropathy (n = 8), IgA nephropathy (n = 4), autosomal-dominant polycystic kidney disease (ADPKD) (n = 4), crescentic glomerulonephritis (n = 4), IgA nephropathy (n = 4 ), lupus nephritis (n = 6), focal segmental glomerulosclerosis (FSGS) (n = 1), single unit kidney (n = 1) and others (n = 11). Mean donor age was 62.7 ± 3.39 (range 60–70, median 62) years; 62 were men and 85 were women. Living related donors were parents (n = 116), siblings (n = 9), spouse (n = 5), extended family member (n = 15) and grandparents (n = 2) with mean HLA match of 3.12 ± 0.95. The average dialysis duration before RTx was 15 months. Over a mean follow-up of 3.4 ± 1.69 (range 0.24–6.15, median 3.7) years, 1- and 5-year patient survival was 95.7% and 82.6%, and death-censored graft survival was 98.5% and 94.8% for 1 and 5 years, respectively, with SCr of 1.8 ± 0.31 mg/dL. There were 21% (n = 31) of biopsy-proven acute rejection (BPAR) episodes, out of which 11.5% (n = 17) were acute B-cell-mediated rejections (AHR), 3.4% (n = 5) acute T-cell-mediated rejections (ATR), 6.1% (n = 9) were combined acute T-cell- and B-cell-mediated rejections and 3.4% (n = 5) had unexplained interstitial fibrosis with tubular atrophy (IFTA). Survival rates are shown in Kaplan–Meier curves (). The mean SCr of donor was 0.9 mg/dL before donation and 1 mg/dL after donation at one-year follow up.
Recipient and donor characteristics in group 2 (donor age < 60 years)
There were 1310 patients in group 1. There were 1117 males and 193 females, with a mean age of 33.84 ± 11.84 (range 6–70; median 32) years. Original diseases leading to ESRD were CGN (n = 623), DN (n = 100), HTN (n = 100), CIN (n = 76), obstructive uropathy (n = 120), IgA nephropathy (n = 34), ADPKD (n = 39), crescentic glomerulonephritis (n = 16), IgA nephropathy (n = 34), lupus nephritis (n = 24), Alport syndrome (n = 18), FSGS (n = 21), single unit kidney (n = 28) and others (n = 111). Mean donor age was 43.45 ± 9.65 (range 13–59, median 45) years; 361 were men and 949 were women. Living related donors were parents (n = 581), siblings (n = 177), spouse (n = 363), extended family member (n = 158), offsprings (n = 29) and grandparents (n = 2) with mean HLA match of 2.46 ± 1.37 (range 0–6: median 3). The average dialysis duration before RTx was one year. Over a mean follow-up of 3.06 ± 1.72 (range 0.17–6.15, median 3) years, 1- and 5-year patient survival was 93.8% and 83.1%, and death censored graft survival was 96.1% and 89% for 1 and 5 years, respectively, with SCr of 1.58 ± 0.37 mg/dL. There were 16.8% (n = 221) of BPAR episodes, out of which 5.6% (n = 74) were AHR, 3.4% (n = 5) ATR, 7.32% (n = 96) were combined acute T-cell- and B-cell-mediated rejections and 5% (n = 66) had IFTA. Survival rates are shown in Kaplan–Meier curves (). There was no difference in patient, graft survival and rejection rates in two groups. The mean SCr of donors was 0.8 mg/dL before donation and 0.9 mg/dL after donation at one-year follow up.
Discussion
Our studies suggest that in the renal transplant outcome from live donors older than 60 years, it is an acceptable alternative. Our data have implications that expand the LD pool. Our findings directly support recent studies. As opposed to the United States, where live donors are more likely to be spouses and friends of the recipients,Citation9,Citation10 donors are more likely to be blood relatives in India and China, especially their parents.
To ensure the kidney is of excellent quality, donor kidney biopsies were performed at the time of transplant in some studies.Citation11,Citation12 Biopsy is the criterion standard for defining the quality of an older kidney. However, a renal biopsy potentially can injure the kidney, leading to postoperative aneurysm formation, rupture and bleeding.Citation13 When a biopsy is not feasible, the study of the macroscopic anatomy and some donor parameters can be acceptable alternatives.Citation14 In our study, donor kidneys were extensively assessed at the time of donation using imaging and laboratory tests; the results were satisfactory. RTx from marginal donors has survival advantages over dialyzed patients on waiting list.Citation15 The relatively small sample size from single center limits the precision of our estimates. Some confounding factors may be present. The induction and maintenance immunosuppression is variable. When interpreting our findings, it must be remembered that the older donors who represent our study group represent a highly selected group, which likely does not represent the unscreened donor population presenting at any given center. When interpreting our results, readers should consider the inherent limitations of observational studies based on registry data. However, this study represents the patient population in the developing country in its totality, and, to our knowledge, is the largest study of its kind.
There are some limitations for our study: The mean follow up of study is 3 and 3.2 years for two groups. It is reported that differences in graft function between older and standard donors will appear beyond 3–4 years.Citation16 So follow-up is short to conclude graft survival and function are similar between groups. Graft function was evaluated by SCr, which is not an ideal marker. The estimated GFR, for example, modification of diet in renal disease or Chronic Kidney Disease Epidemiology Collaboration would have been better than SCr. Death-censored graft survival rates were found to be better than patient survival. It indicated that death with a functioning allograft is more frequent; 12.9% (n = 19) and 10.9% (n = 144) patients were expired in groups 1 and 2; 4% (n = 6) and 6.48% (n = 85) grafts were lost in group 1 and 2, respectively. The extent of nephrosclerosis in these donors could not be evaluated as we did not perform any renal biopsy of donors. There was no significant difference in blood pressure (130/80 mm/Hg) at one-year follow-up; however, long-term blood pressure or renal function level were not done due to lack of long-term donor follow-up.
More experience is needed to determine the outcome of transplants from older LDs. More studies are necessary to better define comparable quality of different donors' organs and to evaluate the effect of other important donor factors that may affect transplant survival and confound the results, including predonation kidney function, donor blood pressure and diabetes in the donor.
Conclusions
Donor age did not affect patient and graft survival in the 5-year follow up in our study. Age alone seems not to be an exclusion criterion to living kidney donation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rajapurkar MM, John GT, Kirpalani AL, et al. What do we know about chronic kidney disease in India: First report of the Indian CKD registry? BMC Nephrol. 2012;13:10
- Goplani KR, Kute VB, Vanikar AV, et al. Expanded criteria donor kidneys for younger recipients: acceptable outcomes. Transplant Proc. 2010;42(10):3931–3934
- Gill J, Bunnapradist S, Danovitch GM, Gjertson D, Gill JS, Cecka M. Outcomes of kidney transplantation from older living donors to older recipients. Am J Kidney Dis. 2008;52(3):541–552
- Li Y, Li J, Fu Q, et al. Kidney transplantation from living related donors aged more than 60 years: A single center experience. Ren Fail. 2013;35:1251–1254
- Lim WH, Clayton P, Wong G, et al. Outcomes of kidney transplantation from older living donors. Transplantation. 2013;95(1):106–113
- Balachandran VP, Aull MJ, Charlton M, et al. Kidneys from older living donors provide excellent intermediate-term outcomes after transplantation. Transplantation. 2012;94(5):499–505
- Berardinelli L, Pozzoli E, Beretta C, et al. Long-term outcome of living donors older than 60 years. Transplant Proc. 2010;42(4):1111–1113
- Sözen H, Fidan K, Onaran M, Arinsoy T, Dalgiç A. Outcome of the using older donors for kidney transplantation; Gazi University, Ankara experience. Transplant Proc. 2010;42(7):2477–2478
- Lan G, Yang L, Peng L, et al. Long-term results of renal transplant from living donors aged over 60 years. Exp Clin Transplant. 2012;10(5):471–474
- Davis CL. Living kidney donors: current state of affairs. Adv Chronic Kidney Dis. 2009;16(4):242–249
- Haas M, Segev DL, Racusen LC, et al. Arteriosclerosis in kidneys from healthy live donors: comparison of wedge and needle core perioperative biopsies. Arch Pathol Lab Med. 2008;132(1):37–42
- Mancilla E, Avila-Casado C, Uribe-Uribe N, et al. Time-zero renal biopsy in living kidney transplantation: A valuable opportunity to correlate predonation clinical data with histological abnormalities. Transplantation. 2008;86(12):1684–1688
- Inoue T, Satoh S, Saito M, et al. A case of ruptured intra-renal aneurysm caused by renal allograft biopsy [in Japanese]. Hinyokika Kiyo. 2008;54(1):9–12
- Santangelo M, Zuccaro M, De Rosa P, et al. Older kidneys donor transplantation: Five years' experience without biopsy and using clinical laboratory and macroscopic anatomy evaluation. Transplant Proc. 2007;39(6):1835–1837
- Ojo AO, Hanson JA, Meier-Kriesche H. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12(3):589–597
- Noppakun K, Cosio FG, Dean PG, et al. Living donor age and kidney transplant outcomes. Am J Transplant. 2011;11(6):1279–1286