Abstract
To explore the possible impact factors on daytime sleepiness among peritoneal patients from a single center in China. A cross-sectional study was conducted in 98 prevalent peritoneal dialysis (PD) patients using both the Pittsburgh Sleep Quality Index (PSQI) questionnaire of sleep quality and the Epworth Sleepiness Scale (ESS) questionnaire of excessive daytime sleepiness (EDS). Biochemical differences between daytime sleepiness and non-daytime sleepiness population were evaluated, following univariate and multivariable analysis to find the risk factors on sleep disturbance. The prevalence of “poor sleep quality” (PSQI > 5) was 74.49%, while daytime sleepiness (ESS ≥ 9) occurred in 22.45%. Mean PSQI was 9.06 ± 4.60 and EES was 6.31 ± 4.98. Compared to non-EDS cases, patients with ESS ≥ 9 had worse residual renal function (RRF), higher serum creatinine, higher serum magnesium and elevated serum ferritin. In univariate analysis, ESS correlated with serum albumin (r = 0.346, p = 0.015), phosphate (r = 0.313, p = 0.029), magnesium (r = 0.376, p = 0.008) and urinary Kt/V (r = −0.341, p = 0.029). Finally, multivariable linear regression indicated that urinary Kt/V, PSQI and magnesium were independent predictors of ESS score. EDS does exist in PD patients and is associated both with poor nighttime sleep quality and lower RRF. Hypermagnesemia may be a treatable risk factor to improve daytime tiredness.
Introduction
Sleep complaints and sleep disorders had been reported quite prevalent in dialysis cases,Citation1–3 and poor sleep quality was independently linked to higher mortality rate in hemodialysis (HD) patients.Citation4 Due to the lots of pattern of sleep disorder and many modes of dialysis, it was hard to achieve a well-recognized theory to elucidate the data. Comparing with that in HD, fewer studies focused on the sleep disorder in peritoneal dialysis (PD) patients, not to mention works in certain type of sleep, i.e., insomnia, sleep apnea, excessive daytime sleepiness (EDS) and restless legs syndrome. In aged population, EDS was associated high rate of cardiovascular disease,Citation5 and the clinical significant EDS might be more frequent, comparing with aged population. Only two single-center researches investigated this symptom with relative small sample-size in PD patients in latest years.Citation6,Citation7 Thus, by the self-reported instruments, the aim of this study was to identify the prevalence of global and certain aspect of sleep quality in PD patients from eastern China as well as clinical correlates.
Materials and methods
Subjects
From January 2010 to March 2010, we screened 110 PD patients and enrolled 98 from the Dialysis Unit of Changhai Hospital, Shanghai, China. Exclusion criteria were PD history of less than three months, congestive heart failure, liver cirrhosis, severe chronic obstructive pulmonary disease, mental or psychiatric impairment or unwillingness to participate in the study. The study protocol was approved by the local ethics committee, and written informed consent was obtained from each participant. Enrolled patients completed two questionnaires during in hospital dialysis session or while waiting for a consultation. Before starting, the patients had each questionnaire explained, and assistance was also provided during the filling out, if needed.
Questionnaires
For assessment of general sleep quality, the Pittsburgh sleep quality index (PSQI) was used, which had been developed by Buysse et al.Citation8 Furthermore, the Chinese version of the PSQI had also been validated.Citation9 It was a valid and standardized measurement for sleep quality evaluation and it could make a reliable semi-quantification of sleep during the past one month period. The questionnaire contains 19 self-rated questions yielding seven specific patient components. Each component was scored from 0 to 3, yielding a global PSQI score between 0 and 21, with higher scores indicating lower quality of sleep. The obtained data are translated into the overall sleep quality as good (0–5 points) and poor (6–21 points). Although PSQI achieved worldwide good performance of sleep assessment, the index could not indicate the certain type of the prevalence of sleep disorders. Thus some specific questionnaires were introduced into the clinical research of certain type of sleep quality.
In general, daytime sleep function was assessed subjectively by the Epworth Sleepiness Scale (ESS). The ESS asked patients to rank their likelihood of dozing in eight different situations, with “0” indicating “would never doze” and “3” indicating “high chance of dozing”. The total score ranges from 0 to 24, with more than 9 indicating the presence of EDS.Citation10 The validity and reliability of the Chinese version of ESS had also been conducted.Citation11
A review of patients’ charts was carried out within one month of recruitment, and relevant clinical data were also recorded. Adequacy of dialysis was assessed by Kt/V. The total weekly urea Kt/V (total Kt/V) and the normalized protein catabolic rate (nPCR) were analyzed based on 24 hours peritoneal effluent and urine collection. The contribution of total Kt/V by PD (Kt/V PD) and residual renal function (RRF), defined as urinary Kt/V (KRU) were estimated separately. All of the bio-fluid samples were collected according the standard protocol, and the specimen was preserved well under the circumstance of four degree before analyzed by automatic biochemical analyzer (HITACHI 7600; Hitachi Co., Tokyo, Japan).
Statistical analysis
Data was expressed as mean ± SD or as median (25%–75% quartiles), as appropriate. Comparisons between groups were done by an unpaired Student’s t test. Nonparametric data were compared using a Mann–Whitney U test. For normally distributed variables, Pearson’s correlation test was used; for non-normally distributed variables, Spearman’s rank correlation test was used. Factors reaching statistical significance and the traditional risk factors were then included in a multivariable linear regression analysis with stepwise model. All p values were two-tailed, and values of <0.05 were considered to indicate statistical significance. All analyses were performed using the SPSS statistical software for Windows (version 17.0, SPSS Inc., Chicago, IL).
Results
The characteristics of the 98 patients are listed in , with differences between groups shown in . The average age was 57.12 years, and 56.12% were male. The median duration of CAPD was 16 months. The average PSQI score and ESS score for study participants were 9.06 ± 4.60 and 6.31 ± 4.98, separately. Twenty-five patients (25.51%) were classified as good sleepers (PSQI ≤ 5) and 73 patients (74.49%) were bad sleepers (PSQI > 5). The average score of PSQI in good and bad sleepers were 3.50 and 10.86, respectively. Compared with bad sleepers, good ones had significantly higher KRU (p = 0.046). Patients having ESS score ≥9 (indicating the presence of EDS) were 22.45% of the total sample. Compared with patients ESS ≥ 9, patients with EDS had a worse RRF (KRU 0.31 ± 0.30 vs. 0.59 ± 0.40, p = 0.037) significantly higher serum creatinine (1010.73 ± 340.03 vs. 776.16 ± 323.12 mmol/L, p = 0.041), higher serum magnesium (1.09 ± 0.37 vs. 0.91 ± 0.18 mmol/L, p = 0.034) and an elevated serum ferritin (575.45 vs. 332.90, p = 0.010).
Table 1. Characteristics of peritoneal dialysis patients recruited in the cross-sectional study.
Table 2. Characteristics of groups with different sleep quality among the 98 PD patients.
Univariate correlates of PSQI and ESS are listed in . Briefly, PSQI correlated with a low KRU (r = −0.392, p = 0.032), while ESS correlated with serum albumin (r = 0.346, p = 0.015), phosphate (r = 0.313, p = 0.029), magnesium (r = 0.376, p = 0.008) and KRU (r = −0.341, p = 0.029). The relationships between PSQI and KRU, as well as ESS between KRU are illustrated in .
Figure 1. Correlation between Pittsburgh Sleep Quality Index and urinary Kt/V (KRU) (A), Epworth Sleepiness Scale (B) and urinary Kt/V (KRU).
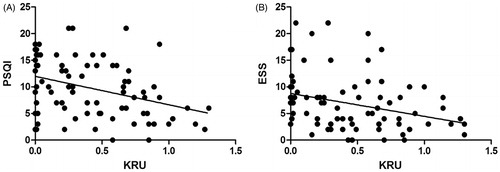
Table 3. Variables correlation with global PSQI score and ESS score.
lists independent predictors of PSQI and ESS. Briefly, PSQI was predicted by KRU (β = −0.406, p = 0.029) and ESS (β = 0.546, p < 0.001), while ESS was predicted by KRU (β = −0.296, p = 0.047), magnesium (β = 0.314, p = 0.019) and PSQI (β = 0.496, p < 0.001).
Table 4. Multivariable linear regression analysis for the global PSQI score and ESS score.
Discussion
This study confirmed previous data suggesting a high prevalence of poor sleep quality amongst patients undergoing PD.Citation12–15 We also found that EDS is associated both with a poor nighttime sleep quality and a lower RRF.
Daytime sleepiness is not studied well in PD patients, but the few papers suggest that the prevalence may vary greatly between populations. Indeed, our finding of a 22% prevalence was similar to that reported by Chen et al.Citation16 (17%) in 710 Taiwanese HD patients, but higher than that reported by Merlino et al.Citation17 (12%) in 883 Italian dialysis patients. Sabbatini et al. reported a much higher prevalence (41%) in 694 Italian HD patients,Citation18 while other studies also reported different prevalence of EDS in HD or mixed patients.Citation19,Citation20 This large variation may reflect both ethnic and cultural differences in subjective reporting, as well as different impacts of dialysis modality and dose, comorbidities and medication use. Meanwhile, the etiology of sleep disorders among dialysis patients are not well understood and are likely to be multifactorial. Old age, smoking, alcohol consumption caffeine intake, pruritus, bone pain, discontinuing dialysis and neuropathy have all been related to insomnia or sleep quality in dialysis patients.Citation1,Citation2,Citation11,Citation17
To these data, our study added the importance of nighttime sleep for daytime wakefulness, as well as the novel relationship between serum magnesium and sleep quality. Sleep apnea and low O2 saturation levels could conceivably be caused by hypermagnesemia due to shallow breathing,Citation21 which may be one explanation. Furthermore, and not surprisingly, we find that patients with better preserved RRF tend to be less tired during the day. Though the NECOSAD study reported an important contribution of RRF to most dimensions of quality of life by the means of another questionnaire, SF-36, involving sleep disorders.Citation22 To the best of our knowledge, it was the first study to find the possible link between RRF and EDS in PD patients. Uremic encephalopathy is well described in ESRD, and daytime sleepiness might share some of these characteristics.Citation23 Preservation of RRF was of clinical relevance in PD, which can bring many benefits, such as reduction in blood pressure and left ventricular hypertrophy, increased sodium removal, improved fluid condition, increased β2-microglobulin clearance and higher serum hemoglobin levels, better nutritional status and decreased circulating inflammatory markers,Citation24 though most of the specific underlying mechanisms are still poorly understood. Recently, Ignace et al. found that a preserved RRF is associated with lower levels of oxidative stress markers in stable PD patients.Citation25 It is possible that lower RRF is the link between sleep disturbance and reduced survival in PD patients. Unlike the data in HD patients,Citation26 we did not find any relationship of inflammation (measured as high-sensitivity C-reactive protein and interleukin 1β) with sleep quality or daytime tiredness (data not shown). This may be explained as RRF was relatively better preserved in PD than HD patients. Latest report indicated that residual diuresis had a beneficial effect on the left ventricular function in HD patients,Citation27 and fluid overload and micro-inflammation were associated with left ventricular hypertrophy.Citation28 Thus we speculated that the better RRF was associated with better left ventricular function, which reduced fluid overload and micro-inflammation. Resulting of lower fluid overload and inflammation status improved EDS quality were found in better RRF ones.
In the other hand, the relationship between calcium–phosphate metabolism and ESS had been also demonstrated in this work. Just like the inflammatory status, the potential link could be found between good RRF and calcium–phosphate balance. Meanwhile the higher phosphate level, the higher risk for arterial calcification and endothelial dysfunction, while would be the source of insomnia, cognitive impairment and daytime sleepiness.
Also of interest was the relationship of ESS with PSQI. It was conceivable that patients with poor sleep quality at night might experience excessive tiredness during the day. Thus, these patients might also require taking a longer daytime nap to compensate for the nocturnal sleep deprivation.Citation12 Recently, ESS had been suggested to reflect sleep apnea,Citation29 a risk factor for cardiovascular morbidity and mortality in PD patients,Citation30 while the complication was potentially treatable. Tang et al. reported improving sleep with transfer to nocturnal cycler-assisted PD.Citation31
Our work has several limitations that should be kept in mind. First, PSQI and ESS were subjective and semi-quantitative measurements. Objective and quantitative tools like comprehensive polysomnography and the Multiple Sleep Latency Test were not performed. Second, certain medications such as beta-blockers, statins, sleep medications and anti-epileptics, which may influence sleep quality, were not controlled. Finally, this cross-sectional study can not reveal the causality in observed relationships.
In conclusion, we report a high prevalence of poor sleep quality in PD patients and the correlation of daytime tiredness with low RRF, poor nighttime sleep quality and hypermagnesemia in PD patients, suggesting that interventions aimed at increasing daytime alertness could be one way to improve the quality of life in PD patients.
Acknowledgments
Authors are grateful to the patients who participated in this study.
Declaration of interest
All the authors declared no competing interests.
This study was supported by Shanghai Nature Science Funding of China (No.07ZR14144). Part of the results from this study was presented in abstract form at the 13th Congress of the International Society for Peritoneal Dialysis in Mexico City, July 23–26, 2010.
References
- Holley JL, Nespor S, Rault R. A comparison of reported sleep disorders in patients on chronic hemodialysis and continuous peritoneal dialysis. Am J Kidney Dis. 1992;19:156–161
- Walker S, Fine A, Kryger MH. Sleep complaints are common in a dialysis unit. Am J Kidney Dis. 1995;26:751–756
- Masoumi M, Naini AE, Aghaghazvini R, Amra B, Gholamrezaei A. Sleep quality in patients on maintenance hemodialysis and peritoneal dialysis. Int J Prev Med. 2013;4:165–172
- Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in hemodialysis patients: Results from the dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transpl. 2008;23:998–1004
- Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults: The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–123
- Bilgic A, Akman B, Sezer S, Arat Z, Ozelsancak R, Ozdemir N. Daytime sleepiness and quality of life in peritoneal dialysis patients. Ther Apher Dial. 2011;15:565–571
- Al-Jahdali H. Prevalence of sleep apnea and excessive day time sleepiness in patients with end-stage renal disease on dialysis. Saudi J Kidney Dis Transpl. 2012;23:251–261
- Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213
- Chen W, Shen YD, Chen R, Ding GX. Investigation on sleep status of college and high school students. Zhonghua Yu Fang Yi Xue Za Zhi. 2005;39:48–50
- Vignatelli L, Plazzi G, Barbato A, et al. GINSEN (Gruppo Italiano Narcolessia Studio Epidemiologico Nazionale), Italian version of the Epworth sleepiness scale: External validity. Neurol Sci. 2003;23:295–300
- Liu GF, Han S, Liang DH, et al. Driver sleepiness and risk of car crashes in Shenyang, a Chinese northeastern city: Population-based case-control study. Biomed Environ Sci. 2003;16:219–226
- Lui SL, Ng F, Lo WK. Factors associated with sleep disorders in Chinese patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2002;22:677–682
- Stepanski E, Faber M, Zorick F, Basner R, Roth T. Sleep disorders in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1995;6:192–197
- Hui DS, Wong TY, Ko FW, et al. Prevalence of sleep disturbances in Chinese patients with end-stage renal failure on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2000;36:783–788
- Li H, Li X, Feng S, Zhang G, Wang W, Wang S. Sleep disorders and its related risk factors in patients undergoing chronic peritoneal dialysis. Chin Med J (Engl). 2014;127:1289–1293
- Chen WC, Lim PS, Wu WC, et al. Sleep behavior disorders in a large cohort of Chinese (Taiwanese) patients maintained by long-term hemodialysis. Am J Kidney Dis. 2006;48:277–284
- Merlino G, Piani A, Dolso P, et al. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transpl. 2006;21:184–190
- Sabbatini M, Minale B, Crispo A, et al. Insomnia in maintenance hemodialysis patients. Nephrol Dial Transpl. 2002;17:852–856
- Hanly P, Gabor J, Chan C, Pierratos A. Daytime sleepiness in patients with chronic renal failure: Impact of nocturnal hemodialysis. Am J Kidney Dis. 2003;41:403–410
- Mucsi I, Molnar MZ, Rethelyi J, et al. Sleep disorders and illness intrusiveness in patients on chronic dialysis. Nephrol Dial Transpl. 2004;19:1815–1822
- Carvalho, B. Respiratory depression after neuraxial opioids in the obstetric setting. Anesth Analg. 2008;107:956–961
- Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT; NECOSAD Study Group. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis. 2003;41:1293–1302
- Parker KP, Bliwise DL, Bailey JL, Rye DB. Daytime sleepiness in stable hemodialysis patients. Am J Kidney Dis. 2003;41:394–402
- Marrón B, Remón C, Pérez-Fontán M, Quirós P, Ortíz A. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl. 2008;73:S42–S51
- Ignace S, Fouque D, Arkouche W, Steghen JP, Guebre F. Preserved residual renal function is associated with lower oxidative stress in peritoneal dialysis patients. Nephrol Dial Transpl. 2009;24:1685–1689
- Chiu YL, Chuang YF, Fang KC, et al. Higher systemic inflammation is associated with poorer sleep quality in stable hemodialysis patients. Nephrol Dial Transpl. 2009;24:247–251
- Ma T, Ding G. Effects of residual renal function on left ventricle and analysis of related factors in patients with hemodialysis. Ren Fail. 2013;35:198–203
- Xu Y, Chen Y, Li D, et al. Hypertension, fluid overload and micro inflammation are associated with left ventricular hypertrophy in maintenance hemodialysis patients. Ren Fail. 2013;35:1204–1209
- Tang SC, Lai KN. Sleep disturbances and sleep apnea in patients on chronic peritoneal dialysis. J Nephrol. 2009;22:318–325
- Tang SC, Lam B, Yao TJ, et al. Sleep apnea is a novel risk predictor of cardiovascular morbidity and death in patients receiving peritoneal dialysis. Kidney Int. 2010;77:1031–1038
- Tang SC, Lam B, Ku PP, et al. Alleviation of sleep apnea in patients with chronic renal failure by nocturnal cycler-assisted peritoneal dialysis compared with conventional continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 2006;17:2607–2616

