Abstract
Background: Residual renal function (RRF) plays a key role in the follow-up of the patients undergoing chronic ambulatory peritoneal dialysis (CAPD). Available methods for measurement of RRF are cumbersome and rarely used, and alternatively, cystatin C-derived equations have been proposed. Methods: Seventy-six adult CAPD patients were recruited. RRF was measured using the 24-hour urea–creatinine clearance method. Serum concentrations of cystatin C were determined. Glomerular filtration rate (GFR) was estimated using the two published equations of Hoek and colleagues, and Yang and colleagues. GFR was also estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. Results: Patients (age range 18–86 years) were on CAPD for a median of 24 months. Average serum concentrations of cystatin C was 5 ± 1.2 mg/L. Average RRF was 0.7 ± 1.6 mL/min/1.73 m2. All three methods significantly overestimated the measured RRF values (Hoek: 4 ± 1.4; Yang: 4.5 ± 1.5; 7.4 ± 4 mL/min/1.73 m2). Based on Bland–Altman plots, all three methods yielded poor agreement with RRF (p < 0.001 for all tests), with Hoek’s equation providing the narrowest limits of agreement [mean difference (limits of agreement): 3.4 (2.9–3.9)] and CKD-EPI the widest [6.7 (5.9–7.5)]. Although the Hoek’s method outperformed CKD-EPI, the within 30 and 50% accuracy rates were unsatisfactory (10.5 and19.7 %, respectively). Conclusions: Cystatin C-derived equations outperform the CKD-EPI formula in approximating the RRF values. Yet, these methods still significantly overestimate the measured RRF and their routine application in clinical practice is not advised.
Introduction
Residual renal function (RRF) has been implicated to have a significant prognostic value in the population of patients on continuous ambulatory peritoneal dialysis (CAPD). A breadth of evidence in the past two decades has shown that RRF predicts mortality in the patients undergoing peritoneal dialysis.Citation1–5 Mechanisms by which preserved RRF contributes to improved patient outcomes include maintenance of fluid balance, norm tension, normal left ventricular function, as well as partaking in clearance of small solutes and toxins of middle molecular weight.Citation6,Citation7 Given the robust association between diminished RRF and patient morbidity, mortality, and quality of life, repeat measurements of RRF encompass an integral part in the management and follow-up of patients on CAPD; preserving RRF, in and of itself, could be viewed as an essential therapeutic target.
In clinical practice, the RRF is usually measured as the arithmetic mean of creatinine and urine clearance which requires 24-h urine collection and a blood draw. Needless to say, the aforementioned process is cumbersome, time-consuming, difficult to monitor, and is therefore subjected to measurement error. For these reasons, a single surrogate marker that could be reliably estimate glomerular filtration rate (GFR) values and be used in place of RRF measurement in clinical practice is desirable. Serum cystatin C has been proposed in this regard.Citation8–13 Cystatin C is a non-glycosylated protein of the cysteine proteinase inhibitors family that is produced by nearly all nucleated cells in the human body.Citation14,Citation15 The production rate of this low molecular weight protein of 13 kDa is nearly constant, and is independent of age, sex, and body mass index(BMI).Citation16,Citation17 Cystatin C is excreted in the bloodstream, is filtered by the renal glomeruli, and is then metabolized by the proximal convoluted tubules.Citation16,Citation18 These unique characteristics make cystatin C potentially the ideal endogenous marker for assessment of renal function.
Previous studies investigating the possible role of cystatin C-derived GFR equations have often favored cystatin C over creatinine-based methods and argued that cystatin C could be used to approximate RRF values with a desirable level of confidence. Hoek and colleaguesCitation8 using serum concentrations of cystatin C showed that a simple linear equation can be used to estimate GFR values surpassing the accuracy of the modification of diet in renal disease (MDRD) creatinine formula. More recently, Yang and colleaguesCitation9 hypothesized that a more complex non-linear relationship may be superior to a linear approach. Accordingly, a hyperbolic model was derived and was able to more reliably approximate RRF levels compared with both MDRD and Hoek’s equation.Citation9 These preliminary efforts, although promising, need to be replicated in independent samples and their generalizability to the wide spectrum of CAPD patients encountered in general practice should be ascertained. With this aim in mind, the present study was designed and conducted. Cystatin C derived equations of GFR along with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)Citation19 GFR values were calculated. The three methods were then compared against the measured RRF values to determine whether these cystatin C-derived equations are able to outperform conventional creatinine-based methods with regard to RRF estimation.
Methods
Patients
Between April 2012 and March 2013 all the patients receiving CAPD, who visited the nephrology clinic of the Imam Khomeini Hospital Complex (a teaching hospital affiliated with Tehran University of Medical Sciences in Tehran, Iran) underwent initial assessment and were recruited if the following inclusion criteria were met: (1) patient was of at least 18 years of age at the time of recruitment; (2) had not been diagnosed with peritonitis in the four weeks preceding enrollment; (3) had stable general, medical, and laboratory condition in the past weeks preceding enrollment; (4) had not been taking corticosteroids in the past six months; (5) the dialysis routine, frequency of dialysis sessions, and dialysate composition had not been changed in the few weeks preceding enrollment; and (6) thyroid stimulating hormone (TSH) concentrations and serum C-reactive protein (CRP) levels were in the normal range. Prior to enrollment, informed consent was obtained from each participant and was formally recorded by the interviewing physician. In the present study, all procedures dealing with human subjects were conducted in accordance with the standards and guidelines laid down in the latest revision of the Helsinki declaration. Local ethics committee of the Tehran University of Medical Sciences also approved the study protocol.
Physical examination, laboratory assessments, and dialysis adequacy
After recruitment, a detailed medical history was obtained and was recorded using a structured questionnaire. Patients then underwent a thorough physical examination conducted by the interviewing physician. Weight of those patients having only light clothing on was measured using a digital scale and was recorded with 0.1 kg precision. Height was measured using a standard stadiometer and was recorded to the nearest 0.1 cm. BMI was then calculated as weight in kilograms divided by height in squared meters (kg/m2). In the same visit, 24-h urine samples and also 24-h dialysate were collected. A venous blood sample was also drawn from each individual and was sent to hospital laboratory for biochemical analysis. Serum and urine creatinine were assessed using the Jaffe method. No calibration was done for creatinine values. Albumin and urea concentrations were determined by photometric methods using the available commercial kits (ParsAzmun, Karaj, Iran). CRP concentrations were quantitatively determined by turbidimetric methods (Biosystems S.A., Barcelona, Spain).
TSH levels were measured using the enzyme-linked immunosorbent assay (ELISA) method (DiaPlus Inc., North York, Ontario, Canada). Serum concentrations of cystatin C were determined by a particle-enhanced immunoassay technique (Gentian, Moss, Norway). For all patients, a peritoneal equilibrium test was conducted following the standard method described by Pannekeet and colleagues.Citation20 Indices of dialysis adequacy, namely, total, peritoneal, and renal Kt/V urea were also calculated.
Definitions and equations
Residual renal function was calculated according to the equation provided by van Olden and colleagues:Citation21
The derived values where then adjusted for body surface area using the Gehan and George formula:Citation22
Estimated GFR was then calculated by using the CKD-EPI equation in whitesCitation19 as follows:
where κ is 0.9 for males and 0.7 for females, α is −0.411 for males and −0.329 for females, min indicates the minimum of creatinine/κ, or 1 and max indicates the maximum of creatinine/κ, or 1.
To estimate GFR values from cystatin C, two previously published equations were used. In the equation proposed by Hoek and colleagues,Citation8 the GFR in CAPD patients is estimated as follows:
In the Yang’s equation,Citation9 on the other hand, a hyperbolic function of Sinh is employed:
Assessment of level of agreement between RRF and each of the estimated GFR equations was conducted using the Bland–Altman plotCitation23 by plotting the mean difference against arithmetic mean of the RRF and estimated GFR values. Limits of agreement were defined as ±1.96 SD from mean difference. The within 30% and within 50% accuracy rates were defined as the probability of estimated values to fall within ±30% or ±50% neighborhood of the true RRF.
Statistical analysis
All analyses were performed using the Statistical Package for Social Sciences (SPSS) version 20 (IBM Corp., New York, NY). Continuous variables are presented as mean ± standard deviation (SD) unless specified otherwise. Categorical variables are presented as proportions. Comparison of continuous variables across categories was done using an independent t-test. Correlation between serum cystatin C/serum creatinine with clinical variables and variables of dialysis adequacy were investigated using the Pearson product moment correlation method or Spearman’s rank correlation where any of the variables failed to meet the assumption of normality.
In all tests, a p value of less than 0.05 was considered statistically significant.
Results
In the initial sample, 11 patients (12.6%) had zero RRF and were thus excluded from the analysis. Baseline characteristics of the remaining 76 participants are summarized in . Age of the patients ranged from 19 to 86 years old. Women comprised 51% of the study sample. The most frequent primary kidney disease should be cause of renal failure was diabetes (n = 38, 50.0%), followed by hypertension (35.6%). Patients were on CAPD for a median of 24 months (ranging from 3 to 84 months). Mean serum creatinine and serum cystatin C were 7.8 mg/dL and 5.0 mg/L, respectively. Cystatin C and also creatinine levels did not significantly differ between the two sexes (p = 0.761 and 0.498, respectively).
Table 1. Baseline characteristics of study participants.
According to the peritoneal equilibration test, 54.0% of the patients were defined as high transporters. The proportion of high average, low average, and low transporters were 27.6, 14.5, and 3.9%, respectively. Analysis of dialysis adequacy revealed a mean total Kt/V urea of 2.0. Peritoneal and renal indices were 1.5 and 0.5, respectively. The mean average RRF of the patients adjusted for body surface area was 0.7 ± 1.6 mL/min/1.73 m2. Cystatin C-derived GFR estimates and also CKD-EPI significantly overestimated the RRF; mean values for CKD-EPI, Hoek’s, and Yang’s equations were 7.4, 4.1, and 4.5 mL/min/1.73 m2, respectively.
Results of the correlation analysis are presented in . Results of the correlation analysis are presented in . Cystatin C significantly correlated with RRF in only males but not females. In all other tests, no correlation with age, BMI, serum CRP, serum TSH, serum albumin, Kt/V urea (total, peritoneal and renal), and RRF was documented (p > 0.05 in all tests). Age significantly correlated with serum creatinine in both males and females. In females, serum creatinine showed negative correlations with Kt/V urea peritoneal, renal, total, and RRF; however, for the Kt/V renal and RRF the association did not reach statistical significance. In males, only serum creatinine and Kt/V renal significantly correlated, albeit there was also a trend towards significant correlations between serum creatinine with Kt/V total and RRF (). The findings from Bland–Altman plots are delineated in and . Agreement analysis revealed that both estimation methods result in a significant overestimation of true RRF values (p < 0.001 for both). Of note, the mean difference for CKD-EPI was twice as large as the Hoek’s estimates (6.7 vs. 3.4 mL/min/1.73 m2) indicating a poorer level of agreement for CKD-EPI. This was also evident in the difference-against-mean plot which showed a wider scatter of the data for CKD-EPI compared with cystatin C estimates. The within 30% and with 50% accuracy rates for Hoek’s or Yang’s equations were also higher than those observed for CKD-EPI ().
Figure 1. Bland–Altman scatter plots between RRF and GFR estimations methods of (A) CKD-EPI, (B) Hoek’s, and (C) Yang’s. Notes: Horizontal lines delineate mean difference along with upper and lower limits of agreement. RRF, residual renal function; GFR, glomerular filtration rate; CKD-EPI, chronic kidney disease epidemiology collaboration.
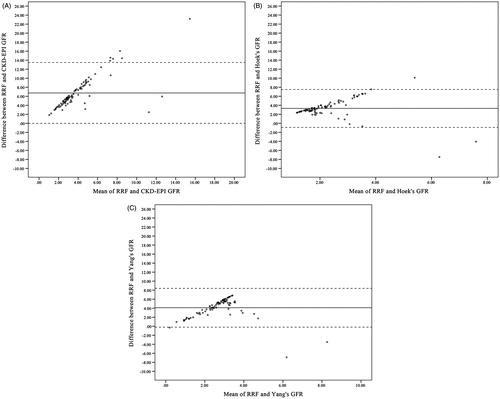
Table 2. Correlation analysis for cystatin C and serum creatinine with clinical parameters and indicators of dialysis adequacy.
Table 3. Agreement analysis between estimated GFR values of cystatin C and CKD-EPI and residual renal function.
Discussion
In the present study, clinical utility of cystatin C-based GFR equations in predicting measured RRF values in a sample of 76 CAPD patients were investigated. Herein, we showed that both Hoek’s and Yang’s equations outperform creatinine-based CKD-EPI formula and produce narrower limits of agreement. Of note, however, both methods still generated values significantly larger than those obtained with the creatinine-urea clearance method, hence overestimating RRF. Previous studies have largely yielded results further confirming that the production and clearance of cystatin C assumes a steady path independent of demographic and clinical features of the population being studied.Citation11,Citation15,Citation16 In our study of CAPD patients, cystatin C levels were not influenced by age, BMI, or inflammation, but influenced by sex. Some studies have also shown that cystatin C is influenced by factors, such as age, BMI, sex, and high concentrations of C-reactive protein.Citation24,Citation25
It has been long shown that cystatin C is superior or at least equivalent to creatinine for estimation of GFR in normal pediatric and adult populations.Citation16,Citation26,Citation27 In the past few years, the possibility of using cystatin C to estimate RRF in CAPD patients has attracted some attention. In one of the earliest efforts, Hoek and colleagues tested the hypothesis that whether a simple equation can reliably estimate GFR values measured using creatinine and urea clearance.Citation8 In a sample of 95 CAPD patients, a linear equation was developed and was subsequently validated on 48 independent subjects.Citation8 Indeed, the equation was shown to be superior to MDRD formula and was advised to be used instead.Citation8 In concert with our findings, they too found that the creatinine-based equation of MDRD, significantly overestimates measured GFR (6.0 vs. 2.9 mL/min/1.73 m2).Citation8 Hoek’s equation, however, when applied to our sample, did also overestimate RRF values, albeit less than CKD-EPI and also Yang’s equations.
Carter and colleaguesCitation10 employed Hoek’s equation to compare estimated cystatin C-derived GFR values with 51Chromium-labeled Ethylenediaminetetraacetic acid (EDTA) clearance method. In a sample of 28 patients undergoing CAPD, it was shown that Hoek’s formula is as precise as 24-hour creatinine/urea clearance but tends to underestimate 51Chromium-labeled EDTA measured GFR by about 20%. In a mixed sample of CAPD and automated peritoneal dialysis (APD), Ros and colleaguesCitation11 compared cystatin C and creatinine in terms of RRF prediction. According to their findings, both method yielded identical results and the area under the curve for both methods were comparable.Citation11 Similar findings of equivalency of both estimation methods have been replicated by Mulay and colleagues.Citation28
In a sample of 120 CAPD patients, Yang and colleaguesCitation9 took advantage of a hyperbolic function to estimate GFR values from cystatin C. The derived formula was superior to both MDRD and Hoek’s equations, when validated in an additional sample of 40 patients.Citation9 The reported within 30% and 50% accuracy for the formula were 57.5 and 77.5%, respectively.Citation9 However, we were not able to replicate these findings. We showed herein that Yang’s formula significantly overestimates RRF values and its accuracy is although better than CKD-EPI, is worse than Hoek’s. The corresponding values for within 30 and 50% accuracy rates in our study were 9.2 and 11.8% for the Yang’s formula indicating that the excellent performance of the equation in the original article might be sample dependent.
While an overestimation of about 3.4 mL/min/1.73 m2 (the smallest difference achieved in this study by using Hoek’s equation) is negligible in a population of patients with normal GFR, the same inference cannot be drawn in the patients undergoing CAPD. This relatively large error is likely due to sample-dependency of the derived formulas. With this in mind, if we had developed a new set of equations specifically tailored for our sample, although it would have fitted the data better with narrower margins of error, it probably would have failed to pass the external validity test when applied to independent populations. Therefore, inclusion of a large sample of patients with a wide range of clinical characteristics, dialysis program, and RRF values preferably from multiple dialysis centers is advised in order to derive an equation capable of approximating measured RRF values with reasonable margins of error.
In the present study, total Kt/V was 2.0 which is comparable to the figures recorded by Yang et al.Citation9 in the CAPD patients (2.2 and 2.1 for modeling and validation groups, respectively). Yang et al.Citation9 showed that while cystatin C concentrations do not correlate with peritoneal Kt/V, serum creatinine concentrations, on the other hand, do so. A similar observation was also made here; peritoneal Kt/V did not correlate with cystatin C, yet, was negatively correlated with creatinine. Congruent with these observations, it has been demonstrated that although cystatin C is in fact able to excrete into peritoneal dialysis fluid in a pattern similar to that of creatinine, the transferred amount is minute and far less than the creatinine, rendering it negligible from a clinical standpoint.Citation29
A number of limitations in the present study deserve to be mentioned. First, the renal clearance rate of specific radioisotopes, such as inulin, iothalamate, or iohexol have been traditionally served as the “gold standard” for the measurement of GFR.Citation30 Although this method is the most accurate, it is costly, labor-intensive, and rarely used in clinical practice.Citation26,Citation27 In the present study, radioisotope clearance was not performed; hence relies on the 24-hour creatinine/urea clearance for the measurement of RRF. It is of note, however, as described by Olden and colleagues,Citation21 in the patients undergoing CAPD, the RRF values calculated using the two methods closely correlate and creatinine/urea clearance can be used interchangeably in place of gold standard without introducing significant bias into the results. Second, it has been suggested that even small disturbances in thyroid function are able to alter cystatin C production; the patients with hypothyroidism have lower cystatin C levels whereas in hyperthyroidism the serum cystatin C concentrations increase. To negate the influence of imposed bias in this regard, we only recruited those patients with normal TSH values. Indeed, correlation analysis confirmed that in the recruited sample of normal TSH, cystatin C levels do not correlate with TSH (r = 0.021, p = 0.858).
In conclusion, our study demonstrates that when the 24-hour creatinine/urea clearance is considered the gold standard, the cystatin C-based equation outperforms creatinine-based equations to estimate RRF. Yet, given the significant overestimation with even cystatin C, thus far, no endogenous marker has proved to be a reliable substitute for the 24-hour urine collection in RRF assessment. Further studies are required before cystatin C-based estimates could replace creatinine/urea clearance measured RRF in clinical practice with a reasonable level of accuracy.
Declaration of interest
This study was part of a MD thesis supported by Tehran University of Medical Sciences. The present study was supported by Tehran University of Medical Sciences (grant no.:18144).
References
- Maiorca R, Brunori G, Zubani R, et al. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant. 1995;10(12):2295–2305
- Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999;33(3):523–534
- Szeto CC, Wong TY, Leung CB, et al. Importance of dialysis adequacy in mortality and morbidity of chinese CAPD patients. Kidney Int. 2000;58(1):400–407
- Rocco M, Soucie JM, Pastan S, McClellan WM. Peritoneal dialysis adequacy and risk of death. Kidney Int. 2000;58(1):446–457
- Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol. 1996;7(2):198–207
- Bargman JM, Golper TA. The importance of residual renal function for patients on dialysis. Nephrol Dial Transplant. 2005;20(4):671–673
- Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726–1732
- Hoek FJ, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT. Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant. 2007;22(6):1633–1638
- Yang Q, Li R, Zhong Z, et al. Is cystatin C a better marker than creatinine for evaluating residual renal function in patients on continuous ambulatory peritoneal dialysis? Nephrol Dial Transplant. 2011;26(10):3358–3365
- Carter JL, Lane CE, Fan SL, Lamb EJ. Estimation of residual glomerular filtration rate in peritoneal dialysis patients using cystatin C: Comparison with 51Cr-EDTA clearance. Nephrol Dial Transplant. 2011;26(11):3729–3732
- Ros S, Bajo A, del Peso G, et al. Cystatin C as marker of residual renal function in patients on peritoneal dialysis: relation with parameters of peritoneal function. J Nephrol. 2007;20(4):468–473
- Delaney MP, Stevens PE, Al Hasani M, Stowe HJ, Judge C, Lamb EJ. Relationship of serum cystatin C to peritoneal and renal clearance measures in peritoneal dialysis: A cross-sectional study. Am J Kidney Dis. 2008;51(2):278–284
- Khorgami Z, Abdollahi A, Soleimani S, Ahamadi F, Mahdavi-Mazdeh M. Relationship between serum cystatin C and creatinine or dialysis adequacy in patients on chronic maintenance hemodialysis. Nephro-Urol Monthly. 2013;5(2):733–735
- Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34
- Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function – A review. Clin Chem Lab Med. 1999;37(4):389–395
- Laterza OF, Price CP, Scott MG. Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem. 2002;48(5):699–707
- Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Int Med. 2007;147(1):19–27
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. New Engl J Med. 2005;352(20):2049–2060
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612
- Pannekeet MM, Imholz AL, Struijk DG, et al. The standard peritoneal permeability analysis: A tool for the assessment of peritoneal permeability characteristics in CAPD patients. Kidney Int. 1995;48(3):866–875
- van Olden RW, Krediet RT, Struijk DG, Arisz L. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1996;7(5):745–750
- Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep 1. 1970;54(4):225–235
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310
- Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421
- Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660
- Kazama JJ, Kutsuwada K, Ataka K, Maruyama H, Gejyo F. Serum cystatin C reliably detects renal dysfunction in patients with various renal diseases. Nephron. 2002;91(1):13–20
- Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40(2):221–226
- Mulay A, Biyani M, Akbari A. Cystatin C and residual renal function in patients on peritoneal dialysis. Am J Kidney Dis. 2008;52(1):194–195; author reply 5–6
- Al-Wakeel JS, Hammad D, Memon NA, Tarif N, Shah I, Chaudhary A. Serum cystatin C: A surrogate marker for the characteristics of peritoneal membrane in dialysis patients. Saudi J Kidney Dis Transplant. 2009;20(2):227–231
- Swan SK. The search continues – An ideal marker of GFR. Clin Chem. 1997;43(6):913–914

