Abstract
Amikacin (AK) is an antibacterial drug, but it has remarkable nephrotoxic and ototoxic side effects due to increase in reactive oxygen radicals. This study was established to determine the possible protective effects of alpha-lipoic acid (ALA), a powerful antioxidant, on AK-induced nephrotoxicity. Three different groups of rats (n = 6) were administered saline (control), AK (1.2 g/kg, intraperitoneally), ALA (100 mg/kg, p.o.) and AK combination (ALA one day before the AK for five days). Renal function, oxidative stress markers and histological changes were evaluated at the end of the experiment. Malondialdehyde was increased as an indicator of free radical formation in AK-induced group and decreased with ALA treatment. While catalase activity was increased significantly, superoxide dismutase and glutathione peroxidase activities were not statistically significant increased with ALA treatment. The result showed that AK enhanced levels of urea, creatinine and blood urea nitrogen in serum significantly. Administration of ALA reduced these levels of biochemical markers. Histopathological observations were confirmed by biochemical findings. In conclusion, ALA is suggested to be a potential candidate to ameliorate AK-induced nephrotoxicity.
Introduction
Aminoglycoside antibiotics are used in several infections; including Gram-negative aerob bacteria and Gram-positive Staphylococcus aureus. They have beneficial effects such as wide antibacterial efficacy and low cost.Citation1 Aminoglycosides are bactericidal as a result of inhibition of protein synthesis and changed integrity of the bacterial cell membrane.Citation2 Amikacin (AK) is a semi-synthetic derivative of aminoglycosides. It is obtained by acetylation from a natural medicine of the kanamycin and resistant to bacterial enzymes that inactivate natural aminoglycosides. Therefore, it is the most broad-spectrum among aminoglycoside antibiotics.Citation3 Because of these features, they are more preferred. The most important and critical side effect of AK is dose-dependent and reversible nephrotoxicity that restrains the treatment of patients. Recent studies demonstrate that reactive oxygen species have a role in the pathogenesis of AK nephrotoxicity.Citation4,Citation5 Alpha-lipoic acid (ALA) is a naturally occurring dithiol compound that functions as an essential cofactor for mitochondrial enzymes.Citation6 When applied regularly, ALA accumulates in tissues and is converted to dihydrolipoic acid (DHLA) by lipoamide dehydrogenase. In recent studies, both ALA and DHLA were found biologically active.Citation7 ALA reduced superoxide radicals by metal-chelating activity and regenerated other natural antioxidants, such as vitamin C or vitamin E from their radical or inactive forms. Recently, ALA was suggested as an effective antioxidant and has been reported to protect against various drug-induced toxicities.Citation8–12 This study was established to evaluate the possible renoprotective effect of ALA via its antioxidant properties in rats with AK-induced nephrotoxicity. The investigation was performed using histopathological changes and biochemical parameters; including malondialdehyde (MDA), serum urea, blood urea nitrogen (BUN) and serum creatinine (Cr), which are used to observe the development and extent of renal tubular damage in rats due to oxidative stress. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) are antioxidant enzymes that prevent the tissues from oxidative damage. These enzyme activities must be increased in antioxidant treatment groups.
Materials and methods
Experimental conditions
Female Wistar rats weighing 250–300 g were placed in a temperature- (21 ± 2 °C) and humidity-controlled (60 ± 5%) room in which a 12:12 h light:dark cycle was maintained. The rats were distributed into three groups (n = 6): (1) control group, which was applied saline oral gavage for five days; (2) AK group, injected intraperitoneally (i.p.) with 1.2 g/kg AK (Amikozit 500 mg flk, Eczacıbası Corp, Istanbul, Turkey) in a single dose;Citation13 (3) AK + ALA group, injected i.p. single dose of 1.2 g/kg AK plus 100 mg/kg oral gavage ALA (Thioctazid 600 mg tab, Meda Pharma, Istanbul, Turkey) one day before the AK for five days.Citation14 Twenty-four hours after the last administrated dose of ALA, at the end of the experiment, the blood sample was collected, both kidneys were quickly removed under 90 mg/kg ketamine (Alfamin, Alfasan IBV) and 10 mg/kg xylazine (Alfazin, Alfasan IBV) anesthesia and divided equally into two longitudinal sections. One half was placed in formaldehyde solution for routine histopathological examination by light microscopy. The other half of the kidney tissue was homogenized and kept at −80 °C for biochemical evaluations (MDA, SOD, CAT, GPx). Blood samples were extracted to determine the serum levels of urea, BUN and Cr. All experiments in this study were performed in accordance with the guidelines for animal research from the National Institutes of Health and were approved by the Committee on Animal Research at Suleyman Demirel University, Isparta (15/08/2013-04).
The preparation of the tissue samples and biochemical analysis
At the end of five days, all groups were decapitated under anesthesia (ketamine HCl 90 mg/kg + xylazine HCl 10 mg/kg). For biochemical analyses, tissue samples from the kidney were collected. Kidney tissues were homogenized in a motor-driven tissue homogenizer (IKA Ultra-Turrax T25 Basic; Labortechnik, Staufen, Germany) with phosphate buffer (pH 7.4). Unbroken cells, cell debris and nuclei were sedimented by centrifugation at 10,000 g for 10 min. The levels of protein, MDA, and the activities of SOD, GPx, and CAT were determined in the supernatants. Determination of SOD activity was estimated in the supernatant according to the method described by Sun et al.,Citation15 and its activity is expressed as units per milligram protein. Determination of GPx activity was based on the method of Paglia and Valentine, and its activity is expressed as units per milligram protein.Citation16 CAT activity was measured using the method described by Aebi and is expressed as kilo units per gram protein.Citation17 Protein levels in the homogenate and supernatant were determined according to the method of Bradford.Citation18 The MDA levels in tissues were determined from the homogenate by following the double heating method of Drapper and Hadley.Citation19 The concentration of MDA is expressed as micromoles per gram protein (µmol/mg protein) in the kidney. An autoanalyzer (Olympus AU 2700, Melville, NY) was used to determine the activities of SOD, and a spectrophotometry (Shimadzu UV-1601; Shimadzu, Kyoto, Japan) was used to estimate the activity of GPx, CAT and levels of MDA.
Histological determination
Right kidneys were removed from each rat and divided into the lobes after cleaning. The lobes were fixed in 10% neutral buffered formalin and embedded in paraffin. The paraffin-embedded blocks were cut to 4–5 μm and stained with hematoxylin and eosin. The slides were then examined using a light microscope (Olympus BX50) and photographed. All histological evaluations were made twice under blind conditions (without knowledge of the treatment) and histological changes were scored as follows:
score (negative score): no structural damage
score (one positive score): minimal damage
score (two positive scores): middle damage
score (three positive scores): severe damage according to research article of Abdel-Wahhab et al.Citation20
Statistical analysis
For statistical analysis, normality was investigated, and it was shown that some values of the parameters did not fit to the normal distribution. Therefore, as stated by Abdel-Wahhab et al.,Citation20 considering the small number of cases, non-parametric Kruskal–Wallis test, Mann–Whitney U test and chi-square test were used to compare groups. Differences were considered as significant for p < 0.05. All results are expressed as mean ± SEM.
Results
MDA, SOD, CAT, and GPx in the kidney
There were statistically significant differences between all groups (p < 0.05). MDA levels, the marker of oxidative damage in kidney tissue, were enhanced in AK group compared to the control group, but it was not significant. In ALA-treated group, MDA levels were significantly reduced compared to the AK group (p < 0.05). In the antioxidant enzymes, SOD and CAT activities were significantly decreased in AK group compared with control group (p < 0.001 and p < 0.05, respectively), and only CAT activity increased in ALA-treated group compared with the AK group significantly (p < 0.001). There was no significant improvement in GPx antioxidant enzyme measurements ().
Table 1. The biochemical markers of oxidative stress.
Renal function test
Serum urea, BUN and Cr levels were significantly (p < 0.05) increased in the AK group compared to the control group (). Elevations were significantly reduced in ALA treatment compared to AK group (p < 0.05).
Table 2. Serum renal function tests.
Histopathological changes
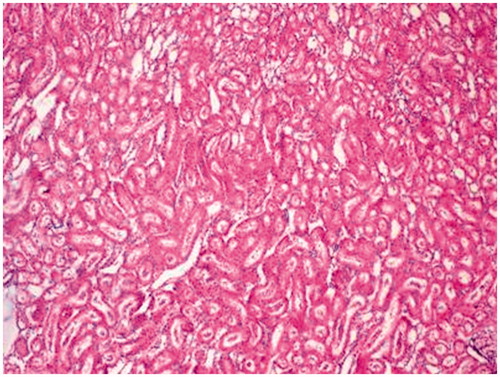
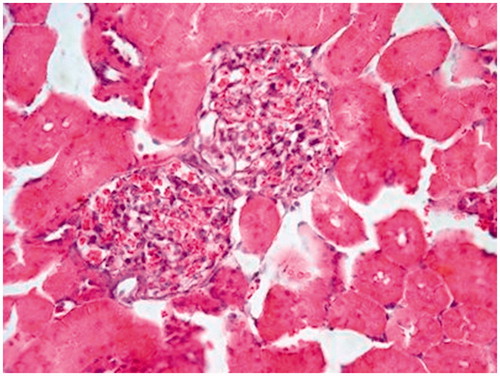
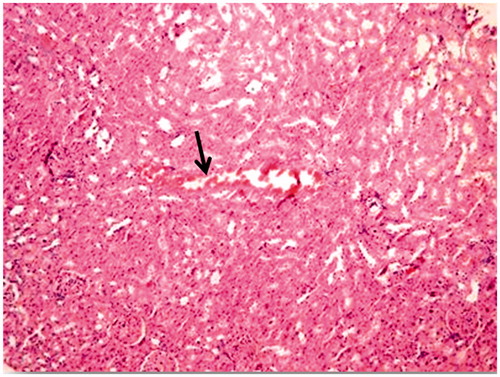
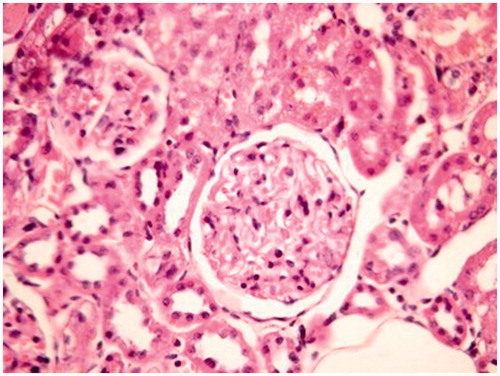
In the evaluation of the kidney tissue sections of control group, there were no histopathological findings observed under the light microscope () ( and ). Glomerular congestion, mononuclear cell infiltration, tubular dilatation, cortical and medullary vascular congestion, proximal and distal tubular degeneration were observed to be statistically significant in AK-administrated group compared with the control group (p < 0.05) ( and ).
Figure 4. Amikacin group. Mononuclear cell infiltration (arrow), tubular dilatation and congestion (arrow head) (×40).
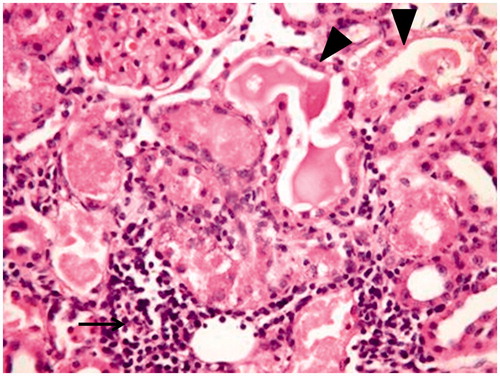
Table 3. Histopathological analysis of kidneys.
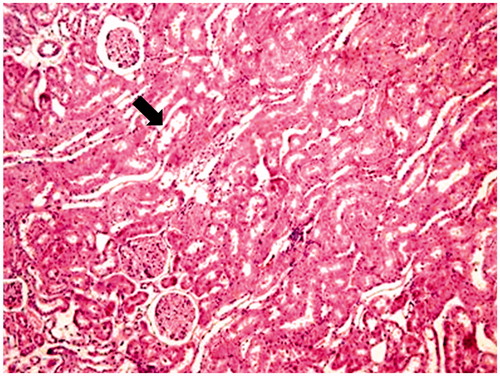
ALA treatment in AK-induced kidney damage, histopathological findings, including especially tubular dilatation, cortex and medulla hemorrhage and proximal and distal tubular degeneration were decreased significantly (p < 0.05) ( and ).
Discussion
Aminoglycoside antibiotics are widely used potent bactericidal drugs. However, nephrotoxicity side effect via oxidant injury limits their effectiveness. The evidence of AK-induced renal damage was demonstrated in several experiments with oxidative stress mechanism in rats.Citation4,Citation5,Citation13,Citation14,Citation21
ALA is widely used as a therapeutic agent for the treatment or prevention of diabetes complications, including polyneuropathy, cataract and nephropathies.Citation22–24 Nowadays, in several studies, antioxidant properties of ALA have been working mainly.Citation9,Citation14,Citation25–28 In addition, ALA contains lipophilic and hydrophilic portions so it has lipophilic and hydrophilic properties, it also removes free radicals, regenerate other antioxidants, connect and facilitate the excretion of heavy metals. It stands out with all of these effects among other major antioxidants.Citation6,Citation29 Chloroquine-induced oxidative damage in kidney ameliorated by the antioxidant properties of ALA at orally 100 mg/kg when compared with other doses (10 and 30 mg/kg).Citation14 This study was designed to check the protective effect of ALA on AK-induced nephrotoxicity at a dose of 100 mg/kg.
ROS can induce lipid peroxidation in the tissues, leading the production of MDA as an indicator of increased lipid peroxidation and tissue damage.Citation30 In this study, AK administration to rats increased MDA levels and ALA treatment significantly decreased MDA production compared with the AK group.
Some studies indicated that superoxide radicals can inhibit GPxCitation31 and CAT activities,Citation32 and singlet oxygen and peroxyl radicals can inhibit SOD and CAT activities.Citation33 The cisplatin-induced toxicity in mice exhibited a significant elevation in MDA accompanying with a significant depletion of CAT, SOD and GPx. ALA may play a renoprotective role on cisplatin-induced nephrotoxicity through antioxidant and antiapoptotic mechanisms combined with the onset of mRNA expression of antioxidant genes.Citation26 In our study, SOD and CAT activities were significantly decreased in AK group compared with the control group; SOD and CAT activities were increased in ALA-treated group compared with AK group, but only CAT activity was statistically significant. There was no significant improvement in GPx activity.
In our study, only AK-induced group increased the level of Cr, urea and BUN (renal damage indicators). Elevations were significantly reduced in ALA-treated group compared to AK group. Nephrotoxicity was manifested by a significant increase in urea and Cr resulted from reduction of glomerular filtration.Citation34 These results are in agreement with the previous reports on AK-induced nephrotoxicity.Citation4,Citation35
Aminoglycosides are excreted from renal proximal tubules cells confirmed that aminoglycosides can induce both proximal and distal renal tubular dysfunction by using proton nuclear magnetic resonance spectroscopy of urine.Citation22,Citation36 Histopathological studies strongly support the concept that tubular necrosis is the primary cause of functional toxicity.Citation5,Citation30,Citation35 In this study, biochemical findings were confirmed by histopathological observation. The AK-applied group showed the highest lesions scores of glomerular congestion, mononuclear cell infiltration, tubular dilatation, cortical and medullary hemorrhage, proximal and distal tubular degeneration, which indicate renal tissue damage. This could be due to the formation of highly reactive radicals as a consequence of oxidative stress caused by the AK. All these changes histopathologically reduced in ALA group. The lower cellular infiltration and lower tissue damage in ALA treatment might be associated with its antioxidant properties.Citation9,Citation26,Citation27 These properties were important in its protective effect against oxidative renal damage.
Recent findings indicate that ALA, an antioxidant, exerts a protective role against the development of diabetic nephropathy, and the underlying mechanism may involve effective suppression of the generation of oxidants, reduction of BUN and Cr.Citation29,Citation37 Wongmekiat et al. demonstrated that ALA supplementation attenuates renal injury in rats with obstructive nephropathy with its beneficial antioxidant properties.Citation24 In another study, ALA improves both renal dysfunction, decrease abnormal levels of MDA and GSH during rat renal ischemia/re-perfusion and all of these supported with immunohistochemistry.Citation38
In conclusion, according to our results, ALA is a powerful antioxidant agent reponsible for reducing the levels of MDA, BUN, Cr and increasing antioxidant parameters, which are the indicators of the damage occurring in the kidney. Based on biochemical and histopathological determinations, ALA efficiently ameliorated AK-induced nephrotoxicity and protects kidney tissues by regenerating renal function and balancing the oxidant–antioxidant status. We thought that ALA, used in routine treatment of diabetic complications, especially neuropathy and nephropathy, would have a new indication such as treatment or prophylaxis of aminoglycoside-induced nephrotoxicity. Depending on our results, we suggest that clinicians could consider the administration of ALA during AK therapy.
Declaration of interest
There was no source of funding for the research. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors would sincerely like to thank Dean of Faculty of Medicine, Professor Huseyin Yorgancigil for technical supports.
References
- Begg EJ, Barclay ML. Aminoglycosides-50 years on. Br J Clin Pharmacol. 1995;39:597–603
- Kadurugamuwa JL, Clarke AJ, Beveridge TJ. Surface action of gentamicin on Pseudomonas aeruginosa. J Bacteriol. 1993;175(18):5798–5805
- Poulikakos P, Falagas ME. Aminoglycoside therapy in infectious diseases. Expert Opin Pharmacother. 2013;14(12):1585–1597
- Parlakpinar H, Ozer MK, Sahna E, Vardi N, Cigremis Y, Acet A. Amikacin-induced acute renal injury in rats: Protective role of melatonin. J Pineal Res. 2003;35(2):85–90
- Ozer MK, Asci H, Oncu M, et al. Effects of pentoxifylline on amikacin-induced nephrotoxicity in rats. Ren Fail. 2009;31(2):134–139
- Karaca EG. Lipoik asit: Evrensel antioksidan. AKU J Sci. 2008;8(1):231–245
- Huk-Kolega H, Skibska B, Kleniewska P, Piechota A, Michalski Ł, Goraca A. Role of lipoic acid in health and disease. Pol Merkur Lekarski. 2011;31(183):183–185
- Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19(2):227–250
- Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM, Farrag MMY. The potential protective role of alpha-lipoic acidagainst acetaminophen-induced hepatic and renal damage. Toxicology. 2008;243(3):261–270
- Amudha G, Josephine A, Sudhahar V, Varalakshmi P. Protective effect of lipoic acid on oxidative and peroxidativedamage in cyclosporine A-induced renal toxicity. Int Immunopharmacol. 2007;7(11):1442–1449
- Kang KP, Kim DH, Jung YJ, et al. Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant. 2009;24(10):3012–3020
- Sehirli O, Sener E, Cetinel S, Yüksel M, Gedik N, Sener G. Alpha-lipoic acid protects against renal ischemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2008;35(3):249–255
- Parlakpinar H, Ozer MK, Ucar M, et al. Protective effects of caffeic acid phenethyl ester (CAPE) on amikacin-induced nephrotoxicity in rats. Cell Biochem Funct. 2006;24(4):363–367
- Murugavel P, Pari L. Attenuation of chloroquine-induced renal damage by alpha-lipoic acid: Possible antioxidant mechanism. Ren Fail. 2004;26(5):517–524
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254
- Drapper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431
- Abdel-Wahhab MA, Nada SA, Arbid MS. Ochratoxicosis: Prevention of developmental toxicity by L-methionine in rats. J Appl Toxicol. 1999;19(1):7–12
- Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–1012
- Takaoka M, Ohkita M, Kobayashi Y, Yuba M, Matsumura Y. Protective effect of alpha-lipoic acid against ischemic acute renal failure in rats. Clin Exp Pharmacol Physiol. 2002;29(3):189–194
- Amudha G, Josephine A, Mythili Y, Sundarapandiyan R, Varalakshmi P. Therapeutic efficacy of DL-alpha-lipoic acid on cyclosporine A induced renal alterations. Eur J Pharmacol. 2007;571(2–3):209–214
- Wongmekiat O, Leelarungrayub D, Thamprasert K. Alpha-lipoic acid attenuates renal injury in rats with obstructive nephropathy. Biomed Res Int. 2013;2013:138719
- El-Beshbishy HA, Bahashwan SA, Aly HA, Fakher HA. Abrogation of cisplatin-induced nephrotoxicity in mice by alpha lipoic acid through ameliorating oxidative stress and enhancing gene expression of antioxidant enzymes. Eur J Pharmacol. 2011;668(1–2):278–284
- Hussein A, Ahmed AA, Shouman SA, Sharawy S. Ameliorating effect of DL-α-lipoic acid against cisplatin-induced nephrotoxicity and cardiotoxicity in experimental animals. Drug Discov Ther. 2012;6(3):147–156
- Dwivedi N, Flora G, Kushwaha P, Flora SJ. Alpha-lipoic acid protects oxidative stress, changes in cholinergic system and tissue histopathology during co-exposure to arsenic-dichlorvos in rats. Environ Toxicol Pharmacol. 2014;37(1):7–23
- Feng B, Yan XF, Xue JL, Xu L, Wang H. The protective effects of α-lipoic acid on kidneys in type 2 diabetic goto-kakisaki rats via reducing oxidative stress. Int J Mol Sci. 2013;14(4):6746–6756
- Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Front Pharmacol. 2011;2:69
- Abdelaziz I, Kandeel M. The protective effect of Nigella sativa oil and Allium sativum extract on amikacin-induced nephrotoxicity. Int J Pharmacol. 2011;7(6):697–703
- Blum J, Fridovich I. Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys. 1985;240(2):500–508
- Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257(10):5751–5754
- Escobar JA, Rubio MA, Lissi EA. Sod and catalase inactivation by singlet oxygen and peroxyl radicals. Free Radic Biol Med. 1996;20(3):285–290
- Martínez-Salgado C, López-Hernández FJ, López-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223(1):86–98
- Parlakpinar H, Koc M, Polat A, et al. Protective effect of aminoguanidine against nephrotoxicity induced by amikacin in rats. Urol Res. 2004;32(4):278–282
- Tzovaras V, Tsimihodimos V, Kostara C, Mitrogianni Z, Elisaf M. Aminoglycoside-induced nephrotoxicity studied by proton magnetic resonance spectroscopy of urine. Nephrol Dial Transplant. 2011;26(10):3219–3224
- Wang L, Wu CG, Fang CQ, et al. The protective effect of α-lipoic acid on mitochondria in the kidney of diabetic rats. Int J Clin Exp Med. 2013;6(2):90–97
- Cavdar Z, Ozbal S, Celik A, et al. The effects of alpha-lipoic acid on MMP-2 and MMP-9 activities in a rat renal ischemia and re-perfusion model. Biotech Histochem. 2014;89:304–314