Abstract
Purpose: The aim of this study was to assess the association between metabolic syndrome (MetS) and urolithiasis. Background: Observational studies and reviews suggest an association between the incidence of urolithiasis and the prevalence of MetS. However, individual studies are needed to be gathered to come to a more reliable and precise conclusion. Methods: We searched the Pubmed–Medline and Embase databases up to February 2014 to identify studies related to urolithiasis and metabolic syndrome. Three authors independently extracted information on the study design, the characteristics of the study participants, exposure and outcome assessments, and the method used to control for potential confounding factors. A random-effects model was used for the risk estimates. Results: Five studies were included in the final analysis. Our meta-analysis of five cross-sectional controlled studies identified a significant association between urolithiasis and MetS, with an overall OR of 1.39 (1.14–1.70). Conclusions: Patients with metabolic syndrome have an increased risk of having urolithiasis indicating that it should be assessed as a systemic disorder. However, these observations need to be evaluated using prospective, randomized studies.
Introduction
Metabolic syndrome, also known as syndrome X and insulin resistance syndrome, is the term which consists of a cluster of disease states-glucose intolerance, elevated blood pressure, dyslipidemia, and central obesity. It is responsible for a three-fold increase in the risk of atherosclerotic cardiovascular diseases (CVD) and also increases mortality from CVD, as well as all-causes, in the general population.Citation1 It has become a major problem in public health because the prevalence of metabolic syndrome in adults is around 20–25% throughout the world.Citation2 Various organizations have used different combinations of criteria for defining metabolic syndrome [International Diabetes Federation, World Health Organization, European Group for the Study of Insulin Resistance, US National Cholesterol Education Program (NCEP), American Heart Association]. The majority of the definitions are on the basis of an individual having three or more of the aforementioned five factors.
All the components of metabolic syndrome are frequently observed in the obese population. Assessment of the overall rise of type 2 diabetes, obesity, metabolic syndrome, and stone disease suggests potential correlation between these conditions. As suggested in recent studies, the increased incidence of uric acid stone formation in the obese population may be due to the production of more acidic urine than non-obese patients. In a study evaluating 24-h urinalyses by Taylor and Curhan,Citation3 higher body mass index (BMI) was found to be associated with increased urinary excretion of oxalate, sodium, uric acid, calcium, and phosphorous as well as lower pH. These findings were confirmed by the group from Boston demonstrating that increased BMI, larger waist size, and weight gain correlated with an increased risk of stone episodes.Citation4 Furthermore, in a study conducted by Einohalli et al., urolithiasis was found more prevalent in patients with nonalcoholic fatty liver disease indicating a strong association between urolithiasis and metabolic disorders.Citation5
Several studies have identified an increased risk of stone disease in diabetics.Citation4,Citation6,Citation7 Common pathophysiology among these patients is insulin resistance, which is associated with a reduction in renal ammonium production and low urinary pH, which could lead to the development of uric acid stones and oxalate calcium stones.Citation8 A direct effect of hyperinsulinemia on urinary calcium excretion was also observed under the euglycemic condition,Citation9 which could promote the formation of kidney stones containing calcium. Furthermore, patients with type 2 diabetes have been found to have lower urinary pH independent of the formation of uric acid stones,Citation10 Additionally, low urinary pH has been shown to directly correlate with the number of metabolic syndrome features, and the degree of insulin resistance was also inversely related to urinary pH.Citation11
Current recommendations suggest that patients with urolithiasis should be screened for metabolic syndrome by virtue of close relationship between these disorders, but the strength of this epidemiologic association has not previously been examined systematically. Consequently, we aimed to combine the individual studies to conduct a meta-analysis to assess the relationship between urolithiasis and metabolic syndrome.
Methods
Study selection and data extraction
The PubMed–Medline and Embase databases were searched independently by three investigators to retrieve relevant studies published before February 2014. The search terms comprised the following keywords: metabolic syndrome, urolithiasis, kidney stone. Studies were included in our meta-analysis if they met the following criteria: (1) the study design was observational; (2) the outcome of interest was the prevalence of MetS in patients with urolithiasis; (3) odds ratios (OR) and corresponding 95% confidence intervals (CIs) (or data to calculate them) were reported. For this study, we selected all the publications that had various definitions of MetS from the following panels/organizations including the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III),Citation12 World Health Organization,Citation13 International Diabetes Federation,Citation14 and the American Heart Association.Citation15 The language for searched publications was restricted to English.
Information extracted from an extensive review of each publication included publication data (first author’s last name, time period of the publication, and country of the population studied), type of study design, cohort size, the percentage of MetS in the study population, percentage of urolithiasis in groups with and without metabolic syndrome, definitions of metabolic syndrome, odds ratios with their corresponding confidence intervals (CIs), and all the covariates (if any) being used in the multivariate analyses and modeling. Three reviewers independently conducted the literature search, study selection and data extraction and any discrepancies were resolved through discussion to come to a consensus.
Statistical analysis
Meta-analysis was performed using the fixed effects method or the random effects method, depending on the absence or presence of significant heterogeneity. Statistical heterogeneity among trials was assessed by using Cochran’s Q and I2 statistic, and p-value <0.10 or I2 value >50% was considered to be heterogeneous. The fixed effects method was used to combine the results when statistically significant heterogeneity was absent. When heterogeneity was confirmed, the random effects method was used. Sensitivity analysis was conducted by omitting one study at a time, generating the pooled estimates and comparing with the original estimates. All statistical comparisons were two-sided, and p-value <0,05 was considered statistically significant. All analyses were performed using Comprehensive Meta-analysis Version 2 (Biostat, Englewood, NJ).
Results
Literature search and study characteristics
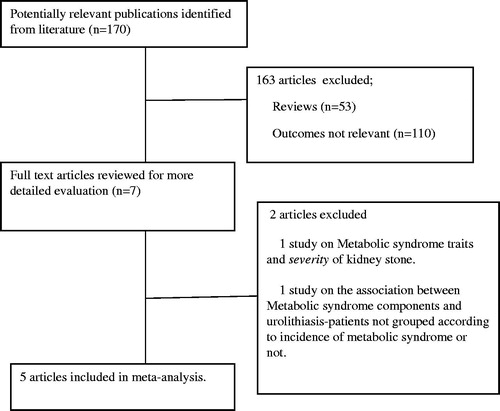
We initially identified 170 studies, either in full publications or abstract forms, using the methodology and the search terms described above. After title and abstract review (excluding 163 articles), seven publications were considered to be relevant to our study subject. Of these seven studies, one was excluded since it was about the association of metabolic syndrome and kidney stone severity, not prevalence. The other study was excluded because it identified the relationship of the components of metabolic syndrome to urolithiasis and did not group patients according to metabolic syndrome presence. Finally, five studies were included in our meta-analysis. The details of the literature search were depicted in .
One study was conducted in the USA, two in Korea and the other two were in Brazil and Italy. The five selected studies contained 169 173 participants (ranging from 740 to 116 536) with 23 976 cases of metabolic syndrome from different populations(two studies in Asia, one study in North America, one study in South America, one study in Europe). The studies were published between 2008 and 2013. Two included studies used both the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III) and American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) criteria, while two studies used AHA/NHLBI and the other study used harmonized criteria (a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; NHLBI; AHA; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity) to define metabolic syndrome. Two studies (West et al., Pinto et al.) used self reports and questionnaire to evaluate the presence or history of urolithiasis, two studies (Ju-Kim et al., Rendina et al.) used ultrasonography and the other one (Jeong et al.) used both ultrasonography and computerized tomography. The characteristics of included studies are presented in .
Table 1. Relevant studies of the relationship between metabolic syndrome and urolithiasis.
Overall analyses on the association of metabolic syndrome and urolithiasis
All studies were cross-sectional. The percentage of patients with MetS was 33.2, 13.1, 11.5, 38.3, and 34%, respectively, while the percentage of urolithiasis was detected higher in patients with metabolic syndrome for all studies. Due to evidence of heterogeneity of the five studies [Q = 27.0663, p-value for heterogeneity = p < 0.0001, I2 = 85.22% (67.26–93.33)], random-effect model was used. Odds ratios (OR) were used to evaluate the association between urolithiasis and MetS. The overall OR in our study was 1.39 (1.14–1.70). summarizes the outcomes of meta-analysis.
Sensitivity analysis and publication bias
We conducted sensitivity analysis by omitting one study at a time, generating the pooled estimates, and comparing the pooled estimates with the original estimates. Omitting any one of five studies concerning MetS and urolithiasis did not produce big difference on the original pooled ORs. The details were shown in . Since the number of studies is less, the interpretation of Funnel plot and/or implementation of Egger's or Begg's test will not be accurate, but significant heterogeneity might indicate that there could be a publication bias. The bias seems to result from the fact that small studies are disproportionately associated with larger effect sizes. In our meta-analysis, we observed that studies with larger cohort tend to have smaller effect size compared to the studies with smaller cohort. This would also reflect the fact that smaller studies are more likely to be published if they have larger than average effects, which makes them more likely to meet the criterion for statistical significance.
Table 2. Sensitivity analysis of five studies included in the meta-analysis.
Discussion
Our systematic review and meta-analysis was designed to estimate the risk of urolithiasis associated with metabolic syndrome. Our results allow us to confirm the strong association of metabolic syndrome with urolithiasis with a 1.4-fold increase.
The pathophysiological mechanism by which the metabolic syndrome increases the risk of urolithiasis remains under debate. The reasons for association between various systemic processes collected within MetS and stone formation cannot be exactly determined, but may include similar metabolic responses and common pathophysiologic mechanisms. Therefore, it is reasonable to discuss the relationship of individual components of metabolic syndrome with urolithiasis.
Hypertension, one of the most important cardiovascular risk factors, has been recognized to be a significant predictor of kidney calcium stones. Borghi et al.Citation21 reported that hypertensive men and women had significantly increased calcium and oxalate excretion and also found that men had increased uric acid excretion as compared to the normotensive cohort. Furthermore, the supersaturation of calcium oxalate was higher in the hypertensive group. Mente et al.Citation22 found significant associations between hypercalciuria and hypertension (multivariate-adjusted odds ratios 2.9). Losito et al.Citation23 found no difference in calcium excretion among stone formers with or without hypertension but they also noted that hypertensive subjects had reduced citrate excretion and urine pH and increased titratable acid excretion when compared with normotensive stone formers. Additionally, as reported in some studies, patients with a history of nephrolithiasis had an increased risk of subsequently developing hypertension.Citation24,Citation25 All these studies indicate that there could be a bidirectional association between hypertension and nephrolithiasis.
Obesity which is a growing health problem worldwide and one of the leading components of MetS has been associated with impaired carbohydrate tolerance and inappropriate calcium response to glucose ingestion. Obesity has been thought to be associated with changes in the environment of urine that facilitate urinary stone formation. In a study by Lee et al.,Citation26 obese individuals were found to have increased amounts of sodium, calcium, and uric acid, whereas their urinary pH was lower compared to non-obese stone formers. Stone analysis revealed that uric acid stone was significantly more commonly found in the obese patients. Causes of low urinary pH include excessive net acid excretion (NAE) and impaired ammonium excretion resulting in poor urine buffering. It has been demonstrated that NAE is significantly elevated in persons with MetS compared with those without the condition.Citation11 Insulin resistance, which is a common entity in MetS, could be a reasonable explanatory factor for the associations between type 2 diabetes mellitus, obesity and renal stone. Abate et al.Citation27 demonstrated that uric acid stone formers were severely insulin resistant. Sakhaee and MaaloufCitation28 reported that as the number of MetS features increased, ammonium excretion was impaired. Furthermore, a more acidic urinary load caused by diet would decrease the amount of urinary citrate, an important inhibitor of kidney stone formation. Cupisti et al.Citation29 found that insulin resistance is also associated with low urine citrate excretion. Diabetic and overweight patients not only tend to have more frequent uric acid stone prevalence but also urine oxalate levels directly correlate with BMI. It has also been reported to be higher in those with diabetes mellitus.Citation30,Citation31
The association between metabolic syndrome components and urolithiasis might also be linked to dietary habits. A low-calcium diet has been demonstrated to be a risk factor for the development of kidney stones.Citation32,Citation33 Low dietary calcium intake has also been reported to be associated with the development of hypertension.Citation34,Citation35 The reasons for this association are unclear but may be due to vitamin D responses. A low-calcium diet stimulates an increase in circulating levels of 1.25(OH)2D3 resulting in an influx of calcium into smooth muscle cells and raised vascular tone, whereas increased calcium consumption does the opposite.Citation36
The metabolic syndrome is basically associated with high risk in CVD, which is linked to chronic inflammation. Inflammation and oxidative stress have also been proposed as playing a major role in kidney stone formation. In light of this, association between stone formation and the development of CVD might be a result of certain common pathological features. Reviews of the recent literature indicate that production of reactive oxygen species (ROS) and development of oxidative stress (OS) may be such a common pathway. In a study conducted by Tsao et al.,Citation37 proinflammatory cytokines, acute inflammation markers, adhesion molecules, urinary microalbumin, myeloperoxidase, 8-hydroxydeoxyguanosine, 3-nitrotyrosine, and monocyte chemoattractant protein were found to be elevated in patients with urolithiasis. In another study, Holoch and TracyCitation38 reported that kidney stone formers in NHANES III had lower levels of serum antioxidant levels including a-carotene, b-carotene, and b-cryptoxanthin. Domingos and SerraCitation39 conducted a study in over 23 000 adult individuals from the 4th Portuguese National Health Survey and found a significant relationship between nephrolithiasis and CVD. Thus, chronic inflammatory status might be another plausible explanation for an increased stone formation in metabolic syndrome.
There are several limitations of this meta-analysis. The studies included in the meta-analysis have cross-sectional nature, which prohibits ascertainment of temporal associations and necessitates further prospective studies. Secondly, there was no enough data for urinary stone formation in studies, so we could only calculate risk ratios generally. We were unable to demonstrate whether MetS increased the risk of a specific type of nephrolithiasis. Thirdly, there was no common method for detecting the presence of urolithiasis. While the presence of urolithiasis was evaluated by self report and questionnaire of the participants in the two studies, ultrasonography and/or computed tomography were used as a diagnostic tool in the other studies. Fourthly, the studies used various versions of MetS definition criteria, which added variation in the interpretation of the results. Fifthly, the number of articles included in meta-analysis might be insufficient but the total number of cases and controls were enough to evaluate and to come to a reliable conclusion. Finally, because the number of studies is less, using the tests to investigate publication bias would not be accurate. Nevertheless, significant heterogeneity could make us think there could be a possible publication bias and despite robustness of the results, this can be assessed as an another deficiency of the study. Despite these limitations, all studies included in the meta-analysis found the same directionality in association between urolithiasis and metabolic syndrome.
Conclusions
Metabolic syndrome is associated with kidney stone disease, and this association suggests that kidney stone disease should be regarded as a systemic disease representing the interaction of multiple risk factors. When treating stone formers, urologists need to be aware of these associations, as they may be able to make the diagnosis of a significant comorbidity that could impact on the patient’s life span and quality of life. Patients should also be informed of these associations and encouraged to make lifestyle modifications to improve their general health and to limit cardiovascular risk.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care. 2005;28:1769–1778
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428
- Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–915
- Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235
- Einollahi B, Naghii MR, Sepandi M. Association of nonalcoholic fatty liver disease (NAFLD) with urolithiasis. Endocr Regul. 2013;47:27–32
- Pak CY, Sakhaee K, Moe O, et al. Biochemical profile of stone forming patients with diabetes mellitus. Urology. 2003;61:523–527
- Lieske JC, Peña de la Vega LS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int. 2006;69:760–764
- Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979
- Shimamoto K, Higashiura K, Nakagawa M, et al. Effects of hyperinsulinemia under the euglycemic condition on calcium and phosphate metabolism in non-obese normotensive subjects. Tohoku J Exp Med. 1995;177:271–278
- Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine composition in type 2 diabetes: Predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–1428
- Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K. Low urine pH: A novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). JAMA. 2001;285:2486–2497
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1:diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553
- Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–1062
- Grundy SM, Brewer Jr HB, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438
- West B, Luke A, Durazo-Arvizu RA, Cao G, Shoham D, Kramer H. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741–747
- Jeong IG, Kang T, Bang JK, et al. Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis. 2011;58:383–388
- Kim YJ, Kim CH, Sung EJ, Kim SR, Shin HC, Jung WJ. Association of nephrolithiasis with metabolic syndrome and its components. Metabolism. 2013;62:808–813
- Filgueiras Pinto Rde S, Almeida JR, Kang HC, Rosa ML, Lugon JR. Metabolic syndrome and associated urolithiasis in adults enrolled in a community-based health program. Fam Pract. 2013;30:276–281
- Rendina D, Mossetti G, De Filippo G, et al. Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: Role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant. 2009;24:900–906
- Borghi L, Meschi T, Guerra A, et al. Essential arterial hypertension and stone disease. Kidney Int. 1999;55:2397–2406
- Mente A, Honey RJ, McLaughlin JM, Bull SB, Logan AG. High urinary calcium excretion and genetic susceptibility to hypertension and kidney stone disease. J Am Soc Nephrol. 2006;17:2567–2575
- Losito A, Nunzi EG, Covarelli C, Nunzi E, Ferrara G. Increased acid excretion in kidney stone formers with essential hypertension. Nephrol Dial Transplant. 2009;24:137–141
- Strazzullo P, Barba G, Vuotto P, et al. Past history of nephrolithiasis and incidence of hypertension in men: A reappraisal based on the results of the Olivetti Prospective Heart Study. Nephrol Dial Transplant. 2001;16:2232–2235
- Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am J Hypertens. 1998;11:46–53
- Lee SC, Kim YJ, Kim TH, Yun SJ, Lee NK, Kim WJ. Impact of obesity in patients with urolithiasis and its prognostic usefulness in stone recurrence. J Urol. 2008;179:570–574
- Abate N, Chandalia M, Cabo-Chan Jr AV, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: Novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392
- Sakhaee K, Maalouf NM. Metabolic syndrome and uric acid nephrolithiasis. Semin Nephrol. 2008;28:174–180
- Cupisti A, Meola M, D’Alessandro C, et al. Insulin resistance and low urinary citrate excretion in calcium stone formers. Biomed Pharmacother. 2007;61:86–90
- Taylor EN, Curhan GC. Determinants of 24-h urinary oxalate excretion. Clin J Am Soc Nephrol. 2008;3:1453–1460
- Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. 2010;183:2244–2248
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 2004;164:885–891
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504
- McCarron DA, Morris CD, Cole C. Dietary calcium in human hypertension. Science. 1982; 217:267–269
- McCarron DA, Morris CD, Stanton JL. Hypertension and calcium. Science. 1984;226:386–393
- Zemel MB. Calcium modulation of hypertension and obesity: Mechanisms and implications. J Am Coll Nutr. 2011;20:428S–435S; discussion 440S–442S
- Tsao KC, Wu TL, Chang PY, Sun CF, Wu LL, Wu JT. Multiple risk markers for atherogenesis associated with chronic inflammation are detectable in patients with renal stones. J Clin Lab Anal. 2007;21:426–431
- Holoch PA, Tracy CR. Antioxidants and self-reported history of kidney stones: The national health and nutrition examination survey. J Endourol. 2011;25:1903–1908
- Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant. 2011;26: 864–868


![Figure 2. Odds ratios and 95% confidence intervals (CIs) of individual studies and of pooled data for the association between Metabolic Syndrome and urolithiasis in all subjects [(Q = 27.0663, p-value for heterogeneity = p < 0.0001. I2 = 85.22% (67.26–93.33)].](/cms/asset/d8123837-27da-438c-880c-c382db0564dc/irnf_a_976133_f0002_b.jpg)