Abstract
Background: Terlipressin improves renal function in some patients with type-1 hepato-renal syndrome (HRS). Renal contrast-enhanced ultrasound (CEUS), a novel imaging modality, may help to predict terlipressin responsiveness. Objectives: We used CEUS to estimate the effect of terlipressin on the renal cortical microcirculation in type-1 HRS. Methods: We performed renal CEUS scans with destruction–replenishment sequences using Sonovue® (Bracco, Milano Italy) as a contrast agent at baseline and after the intravenous administration of 1 mg of terlipressin, in four patients with type-1 HRS. We analyzed video sequences offline using dedicated software. We derived a perfusion index (PI) at each time point for each patient. Results: Patients 1 and 2 had severe presentation and were admitted to the intensive care unit. Both showed a marked increase in PI (+216% and + 567% of baseline) in response to terlipressin. Patients 3 and 4 had less severe presentations and had a decrease in PI (−53% and −20% of baseline) in response to terlipressin. Patients 1, 2, and 4, but not patient 3, responded to terlipressin therapy with a decrease in serum creatinine to <150 µmol/L. Conclusions: CEUS detected changes in renal cortical microcirculation in response to terlipressin and demonstrated heterogeneous microvascular responses to terlipressin. These initial proof-of-concept findings justify future investigations.
Introduction
Terlipressin improves renal outcomes in type-1 hepato-renal syndrome (HRS),Citation1,Citation2 a condition associated with a poor prognosis.Citation3 Terlipressin is thought to redirect blood flow from the splanchnic to the systemic and renal circulations, thereby improving renal function.Citation4 However, only half the patients with HRS respond to terlipressin.Citation5 In the absence of robust predictors of response, the drug is usually initiated and its efficacy is assessed based on the changes in serum creatinine levels over the following few days. This means that some patients are exposed to potentially severe side effectsCitation6 for no benefit and unnecessary cost. Thus, early identification of responders would be desirable.
Contrast-enhanced ultrasonography (CEUS) is a recent imaging modality enabling visualization and quantification of organ perfusion.Citation7,Citation8 Renal cortical microcirculation evaluation has been shown with CEUS in healthy volunteersCitation9,Citation10 and patients undergoing cardiac surgery.Citation11 We hypothesized that CEUS would detect renal microcirculation changes in patients with type-1 HRS following the administration of single dose of terlipressin and conducted a proof-of-concept study.
Methods
The study was approved by the Austin Hospital Human Research Ethics Committee (H2010/04010).
Patients recruitment
Patients admitted to the Austin Health were screened for inclusion criteria between July 2011 and March 2013. Inclusion criteria were established cirrhosis or acute liver failure, type-I HRS. Terlipressin was prescribed by their medical team in charge and all patients provided written informed consent. Exclusion criteria were treatment limitations, hypersensitivity to Sonovue® components, clinically unstable ischemic heart disease (acute coronary syndrome or myocardial infarction within 7 d), acute or chronic (class III/IV) cardiac failure, severe rhythmic disorders (defined as the occurrence of ventricular tachycardia or ventricular fibrillation within 7 d), known left-to-right shunt, severe (>90 mmHg) pulmonary hypertension, pregnancy, and lactation.
Study design
Once enrolled, a baseline renal CEUS examination was performed and perfusion indices (PI) were calculated (detailed procedure below). At the end of the procedure, 1 mg of terlipressin was administered. After 2 h, renal CEUS was repeated under strict identical conditions (same examiner, patient’s position, bed head angulation, ultrasound probe position and angulation, and ultrasound settings).
Additional outcomes of interest included peak and nadir serum creatinine concentration during hospitalization, need for renal replacement therapy, as well as location, vital status, and receipt of liver transplant at days 30 and 90.
CEUS procedure
We used Sonovue® (Bracco, Milano, Italy) as the ultrasound contrast agent (UCA). Low mechanical index renal ultrasound was performed with a Philips IU22® ultrasound machine (United Medical Instruments, San Jose, CA) and a C5-1® probe. A long axis view of the kidney was obtained by placing the transducer probe over the lower back of the subject. Once adequate images of the kidney were obtained, the UCA infusion was started at 1 mL/min using a dedicated syringe pump (VueJect®, Bracco, Milano, Italy). Image depth, focus, gain, and frame rate were optimized at the first examination and held constant throughout the study. During both CEUS examinations, five destruction–replenishment sequences were obtained.Citation12
Sequences analyses
Ultrasound data sets were analyzed offline with dedicated software (VueBox®, Bracco Research, Geneva, Switzerland) (). For each sequence, one region of interest (ROI) was drawn (details for ROI selection and sequence exclusions have been described elsewhereCitation11). Based on the time–intensity curve, the software generated a perfusion index (PI), thought to be proportional to perfusion within the ROI. This PI is the ratio of the relative blood volume (RBV) over the mean transit time (mTT) and is expressed in arbitrary units (a.u.). These parameters have been described elsewhere.Citation12,Citation13 For each patient and each study time, the median value for interpretable measurements was considered for analysis.
Results
Four patients were included in the study (). Two patients had severe presentations and required ICU admission. One required a noradrenaline infusion and renal replacement therapy (prior to terlipressin administration). All patients had severe acute kidney injury.
Table 1. Patients characteristics and outcomes.
Three patients (one, two, and four in ) clinically responded to terlipressin as their serum creatinine decreased to less than 150 µmol/L. All patients were alive at hospital discharge and at day 90. Two patients underwent orthotopic liver transplantation.
CEUS results
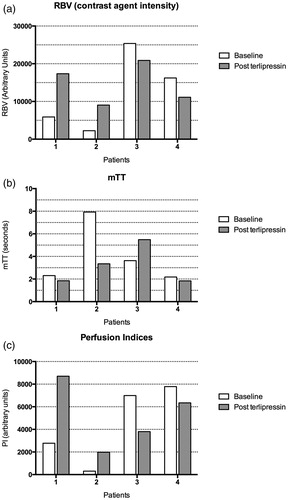
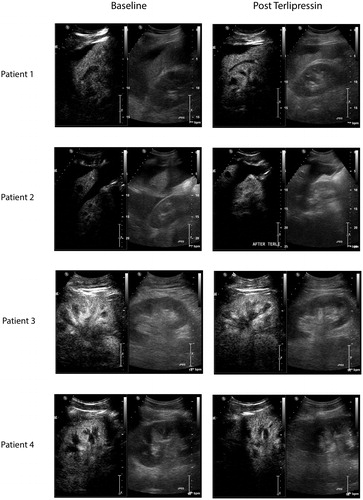
All CEUS studies were well tolerated and no adverse event was noted. CEUS-derived parameters are presented in and still CEUS images obtained before and after terlipressin administration are presented in .
Figure 2. Screenshot of CEUS images. Left side of the panel: images obtained at baseline; right side of the panel images obtained 2 h after intravenous administration of 1 mg terlipressin. Each image is divided into two (left side: contrast-enhanced image and right side: conventional “B mode” ultrasound image for orientation).
Perfusion indices
As shown in , terlipressin administration was associated with a 213% and 548% increase in perfusion indices in patients 1 and 2 (respectively, from 2777 to 8703 a.u. and from 304 to 1974 a.u.). It was associated with a 46% and 19% decrease in patients 3 and 4 (respectively, from 6991 to 3804 a.u. and from 7786 to 6344 a.u.).
Raw parameters
Consistent with the overall changes in PI (), terlipressin administration was associated with an increase in the RBV parameter (consistent with an increase in microcirculation perfusion) in patients 1 and 2 (respectively, from 5882 arbitrary units (a.u.) to 17362 (+195%) and from 2253 to 9034 a.u. (+301%)). Terlipressin administration was associated with a decrease in RBV patients 3 and 4 (respectively, from 25,382 to 20,887 a.u. (−18%) and from 16,218 to 11,113 a.u. (−31%)).
Terlipressin administration was also associated with a 58% decrease in the mTT parameter (consistent with increased perfusion) in patient 2; a 51% (suggestive of decreased perfusion) increase was observed in patient 3 and a non-significant (<25%) change was observed in patients 1 and 4.
Discussion
To the best of our knowledge, this proof-of-concept study is the first to evaluate renal cortical microvascular changes in response to terlipressin in patients with type-1 HRS. We found that CEUS was feasible and well tolerated. We also found that renal cortical perfusion indices as measured by CEUS did not increase in response to terlipressin administration in all patients with type-1 HRS. Indeed, in two patients, with very low perfusion indices at baseline, there was a several fold increase in these values. Both were clinical responders to terlipressin. Conversely, two patients, with higher perfusion indices on baseline, exhibited a modest decrease in their perfusion indices. Among them, only one was a clinical responder to the therapy.
To date, the ability to predict a patient’s response to terlipressin is very limited. Nazar et al.Citation14 reported that only baseline serum bilirubin concentration (with a specificity of 61%) and an increase in mean arterial pressure at day three of treatment were independent predictors of response. A secondary analysis of a randomized controlled trialCitation15 found that baseline creatinine was the only independent predictor of response. Yet, none of these parameters are useful in clinical practice. Hence, a strategy to help determine response to terlipressin after a single dose could limit the exposure to risk and cost. CEUS could be part of such a strategy. Our study, however, is limited by its small number of patients. Indeed, well-documented type-1 HRS is uncommon and most series involving such patients with this condition are of limited size. CEUS-derived parameters are also limited by the high heterogeneity of baseline measurements among patients due to differences in tissue depth and interstitial composition preventing direct patient comparison. This study represents a pilot and feasibility study and justifies further investigations.
Nonetheless, CEUS is a safe, fast,Citation11 non-invasive technique which could enable bedside renal cortical microcirculation quantification in patients with HRS and be also used as a research tool. Such imaging modality could enable to better characterize HRS pathophysiology, an entity which remains poorly understood. Indeed, CEUS might improve current diagnostic criteria for HRS type 1, by introducing a direct evaluation of renal perfusion enabling improved differentiation from other causes of AKI. Further studies involving larger number of patients are required to confirm or refute our preliminary findings.
Conclusions
Renal cortical microcirculation evaluation with CEUS is feasible in patients with type-1 HRS. CEUS-derived perfusion indices can demonstrate dramatic changes after terlipressin therapy and perfusion responses appear variable. Larger studies are necessary to establish the sensitivity and the specificity of CEUS to predict terlipressin responsiveness.
Declaration of interest
The authors report that they have no conflicts of interests. Bracco (Milano, Italy) provided the contrast agent (Sonovue®) free of charge. Bracco Research (Geneva, Switzerland) provided the VueJect™ pump as well as and the VueBox® software free of charge. Both these companies were allowed to read draft of the manuscript before submission but had no influence on its content or decision for submission.
References
- Martin-Llahi M, Pepin M-N, Guevara M, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: A randomized study. Gastroenterology. 2008;134(5):1352–1359
- Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134(5):1360–1368
- Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361(13):1279–1290
- Schrier RW, Niederberger M, Weigert A, Gines P. Peripheral arterial vasodilatation: Determinant of functional spectrum of cirrhosis. Semin Liver Dis. 1994;14(1):14–22
- Fabrizi F, Dixit V, Martin P. Meta-analysis: Terlipressin therapy for the hepatorenal syndrome. Aliment Pharmacol Ther. 2006;24(6):935–944
- Gluud LL, Christensen K, Christensen E, Krag A. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev. 2012;9:CD005162
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473–483
- Tiemann K, Becher H, Lohmeier S. Real-time assessment of tissue perfusion following bubble destruction at low emission-power – First experimental results using power pulse inversion imaging. J Am Coll Cardiol. 2000;35:426A
- Schneider AG, Hofmann L, Wuerzner G, et al. Renal perfusion evaluation with contrast-enhanced ultrasonography. Nephrol Dial Transplant. 2012;27(2):674–681
- Kishimoto N, Mori Y, Nishiue T, et al. Renal blood flow measurement with contrast-enhanced harmonic ultrasonography: Evaluation of dopamine-induced changes in renal cortical perfusion in humans. Clin Nephrol. 2003;59(6):423–428
- Schneider AG, Goodwin MD, Schelleman A, Bailey M, Johnson L, Bellomo R. Contrast-enhanced ultrasound to evaluate changes in renal cortical perfusion around cardiac surgery: A pilot study. Crit Care. 2013;17(4):R138
- Schneider A, Johnson L, Goodwin M, Schelleman A, Bellomo R. Bench-to-bedside review: Contrast enhanced ultrasonography – A promising technique to assess renal perfusion in the ICU. Crit Care. 2011;15(3):157
- Arditi M, Frinking PJ, Zhou X, Rognin NG. A new formalism for the quantification of tissue perfusion by the destruction–replenishment method in contrast ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53(6):1118–1129
- Nazar A, Pereira GH, Guevara M, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51(1):219–226
- Boyer TD, Sanyal AJ, Garcia-Tsao G, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: Relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55(2):315–321