Abstract
To evaluate the association between angiotensinogen (AGT) gene polymorphism and the risk of Henoch–Schönlein purpura (HSP)/Henoch–Schönlein purpura nephritis (HSPN) we searched the eligible studies through Pub Med, Embase, Cochrane, and China National Knowledge Infrastructure (CNKI) databases according to predefined criteria. A random-effects model was used to calculate the combined odds ratios (ORs) and its corresponding 95% confidence interval (CI). Five studies were recruited for the analysis of the association between AGT M235T gene polymorphism and HSP/HSPN risk. M allele was associated with lower risk of HSP in adult (p = 0.050), TT genotype was associated with the susceptibility to HSP in adult (p = 0.039). AGT M235T gene polymorphism was not associated with HSP risk in children. No marked association was observed between AGT M235T gene polymorphism and HSPN risk. No evidence of publication bias was observed. In conclusion, M allele might be a protective factor against the HSP risk in adult, TT genotype might be a risk factor for the susceptibility to HSP in adult. However, further larger studies should be performed in the future.
Introduction
Henoch–Schönlein purpura (HSP) is the most common form of small vessel vasculitis in children.Citation1 Major clinical manifestations include arthritis, non-thrombocytopenic purpura, abdominal pain and renal disease. Although HSP is a self-limited condition that lasts an average of four weeks in most cases,Citation1 a number of HSP cases will progress to be complicated with nephritis. The long-term prognosis of HSP is largely determined by the severity and duration of renal involvement.Citation2,Citation3 To date, no effective approach to prevent Henoch–Schönlein purpura nephritis (HSPN) has been found. Hence, to search for a sensitive biomarker for the onset of HSP/HSPN seems imperative. Genetic factors might be associated with the onset of HSP/HSPN.
The renin–angiotensin system (RAS) mediates the regulation of sodium homeostasis, blood pressure, and inflammation.Citation4 HSP usually occurs due to vasculitis of the small blood vessels, and endothelial cell activation.Citation5 The RAS also plays a role in the modulation of vascular tone and possibly vascular structure either directly or via various factors such as endothelin and nitric oxide.Citation6 Hence, RAS might be involved in the pathogenesis of HSP. Furthermore, angiotensin II promoted the renal tubular and mesangial cell proliferation.Citation6 The local RAS in the kidney also regulates renal cell growth and induces TGF-β production.Citation7 In this sense, RAS is involved in the pathogenesis of renal diseases, including HSPN. Angiotensin II is the main mediator of action of RAS, angiotensinogen (AGT) is involved in the production of angiotensin II.Citation8 Functional polymorphism of AGT was associated with the AGT status. Therefore, we speculated that AGT gene polymorphism might predict the risk of HSP/HSPN through its influence on the level of AGT.
Currently, a number of studies have shown the association between AGT M235T gene polymorphism and HSP/HSPN risk.Citation9–13 However, the results were inconsistent across these studies. An in-depth understanding of this issue may have important clinical implications provided the possibility that AGT M235T gene polymorphism might predict the susceptibility to HSP/HSPN. Meta-analysis is a good way to summarize the available evidence to provide a more robust result. Several previous meta-analysesCitation14,Citation15 reported the association between ACE I/D gene polymorphism and HSP risk. However, the meta-analysis regarding the association between AGT M235T gene polymorphism and HSP/HSPN risk was rare.
With the accumulating evidence, we, therefore, performed this meta-analysis to investigate the association between AGT M235T gene polymorphism and the risk of HSP/HSPN with the aim of providing a much more reliable finding on the significance of this association.
Methods
Search strategy
We searched the published papers that reported the association between AGT M235T gene polymorphism and the risk of HSP/HSPN through May 2014 by using Pub Med, Embase, Cochrane and China National Knowledge Infrastructure (CNKI) databases. No restriction was imposed on search languages. The used search terms were as follows: (1) Henoch–Schönlein purpura, HSP; Henoch–Schönlein purpura nephritis, HSPN; and (2) angiotensinogen, AGT M235T, gene, polymorphism. We also reviewed the reference lists of retrieved reviews and articles. If the same data were enrolled in more than one study, we chose the study with the most complete analysis.
Inclusion and exclusion criteria
Inclusion criteria: (1) case-control study; and (2) the outcome of interest was HSP/HSPN; and (3) a minimum of two comparison groups (HSP group versus Healthy group or HSPN group versus HSP without nephropathy group); and (4) odds ratio (OR) with 95% confidence intervals (CIs) available (data to calculate them).
Exclusion criteria: (1) case reports, editorials and reviews; (2) association between other genes and HSP/HSPN risk; and (3) multiple publications of the same data.
Data extraction and synthesis
We extracted study characteristics from each study. Data were recorded as follows: first author’s last year, publication year, ethnicity of study population, number of cases and controls for AGT M235T genotype. Frequencies of M allele were calculated for case and control groups, from the corresponding genotype distribution. Two authors independently performed the data extraction and quality assessment with any disagreements resolved by discussion.
Statistical analysis
OR was used to measure the association between AGT M235T M/T gene polymorphism and HSP/HSPN risk across studies. Heterogeneity of ORs among studies was tested by using the Q statistic. The I2 statistic, a quantitative measure of inconsistency across studies, was also computed. The combined ORs were calculated using a random-effects model. In addition, 95% confidence intervals (CIs) were also calculated. In order to avoid excessive comparisons, the OR was calculated using three methods: method 1, allele comparison (M allele vs. T allele); method 2, comparing MM homozygous with the other two combinations (MM vs. MT + TT); and method 3, comparing TT genotype with the other two combinations (TT vs. MT + MM). A chi-square test using a web-based program was used to determine whether genotype distribution of the control population reported conformed to Hardy–Weinberg equilibrium (HWE) (HWE; significance level at p < 0.05). Subgroup analysis was conducted in adult and children with HSP/HSPN. Potential publication bias was assessed by Begg’s test and Egger’s test (p < 0.05 was considered significant). All analyses were conducted using STATA version 12.0 (Stata Corp, College Station, TX). p Value < 0.05 was considered statistically significant, except where otherwise specified.
Results
Literature search
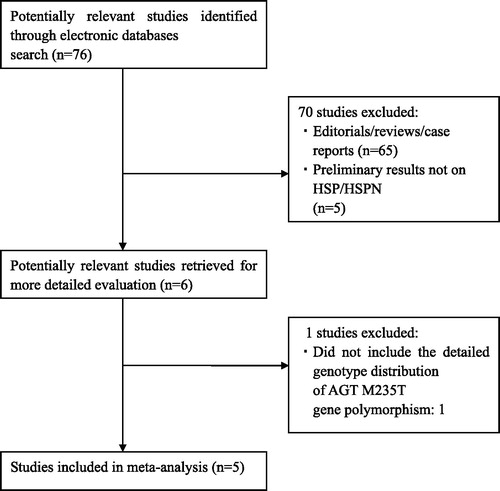
We initially extracted 76 relevant publications from the Pub Med, Embase, Cochrane and China National Knowledge Infrastructure (CNKI) databases. Of these, 70 studies were excluded according to the inclusion and exclusion criteria, five articlesCitation4,Citation6–22 were included in our meta-analysis (). The retrieved data were recorded as follows: first author’s surname, publication year, ethnicity, the number of cases and controls.
Characteristics for included studies
Five studiesCitation9–13 were identified for the analysis of the association between AGT M235T gene polymorphism and the risk of HSP/HSPN (). They were all conducted in Asians. These studies were published between 2006 and 2013. A total of 676 HSP patients and 702 healthy controls were included. Meanwhile, a total of 334 HSPN patients and 342 HSP subjects without renal involvement were recruited. The genotype distributions of the control groups in all the studies were in HWE. The average frequency of the M allele was 35.9% in adult HSP patients and 47.9% in controls. For children, the average frequency of M allele was 58.4% in the HSP patients group and 69.6% for controls. The ratio of HSP/controls for average frequency of M allele in adult was obviously lower compared with that in children (Adult: cases/controls = 0.75; Children: cases/controls = 0.84). The average frequency of the M allele was 30.2% in adult HSPN and 39.6% in controls. For children, the average frequency of M allele was 54.7% in the HSPN and 62.4% for controls. The ratio of HSPN/controls for average frequency of M allele in adult was obviously lower compared with that in children (Adult: cases/controls = 0.76; Children: cases/controls = 0.88).
Table 1. Characteristics of studies evaluating the effects of AGT M235T gene polymorphism on HSP/HSPN risk.
Association of AGT M2357 gene polymorphism with HSP/HSPN
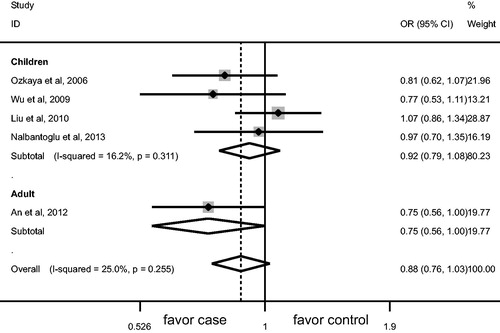
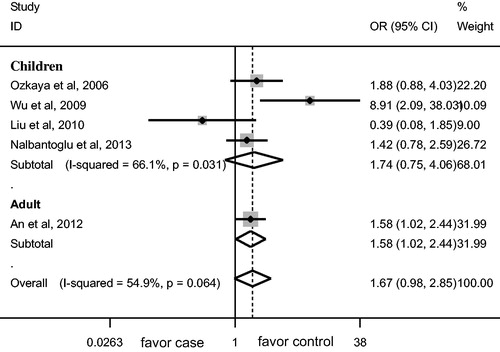
AGT M235T gene polymorphism was not associated with the risk of HSP/HSPN in overall populations and children (). No significant association was observed between AGT M235T gene polymorphism and HSPN risk in adult (). M allele was associated with lower risk of HSP in adult (p = 0.050, , ), TT genotype was associated with the susceptibility to HSP in adult (p = 0.039, , ).
Table 2. Meta-analysis of the association of AGT M235T gene polymorphism with HSP/HSPN risk.
Publication bias
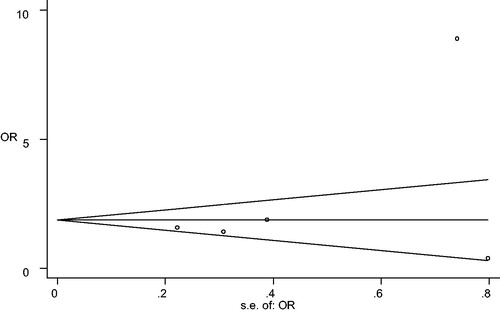
The funnel plot showed no marked asymmetry (). The Begg rank correlation test and Egger linear regression test indicated no significant publication bias across studies (M vs. T: Begg p = 0.327, Egger p = 0.250; MM vs. MT + TT: Begg p = 0.999, Egger p = 0.927; TT vs. MT + MM: Begg p = 0.624, Egger p = 0.366).
Discussion
Increasing attention has been paid to the association between AGT M235T gene polymorphism and the risk of HSP/HSPN. Our meta-analysis showed that M allele was associated with lower risk of HSP in adult, TT genotype was associated with the susceptibility to HSP in adult. AGT M235T gene polymorphism was not associated with HSP risk in children. No marked association was observed between AGT M235T gene polymorphism and HSPN risk. Notably, only one study regarding adult HSP was included, which made our conclusion less robust. Nevertheless, our findings still have implication that TT genotype might be a genetic marker for the onset of HSP in adult.
The age differences might affect the association between AGT M235T gene polymorphism and HSP/HSPN risk. In our study, the ratio of HSP/controls for average frequency of M allele was 0.75 and 0.84 in adult and children, respectively.
The ratio of HSPN/controls for average frequency of M allele was 0.76 and 0.88 in adult and children, respectively. Notably, the ratio in adult is lower than that in children, which is consistent with our findings that M allele conferred protection against HSP risk in adult, TT genotype might be a risk factor for the susceptibility to HSP in adult. However, there was only one study included for the meta-analysis in adult, which made it difficult to draw a robust conclusion for adult. More studies regarding adult are needed.
Several mechanisms may account for the association between AGT M235T gene polymorphism and HSP/HSPN risk. First, the RAS is involved in the progress of several immune-inflammatory diseases.Citation16 Over-activation of the RAS is involved in the initiation of inflammation.Citation16 HSP is a leukocytoclastic vasculitis and is due to a complex series of inflammatory and immunologic processes. The interaction between leukocytes and vascular endothelial cells contributes to the pathogenesis of leukocytoclastic vasculitis.Citation17 Furthermore, RAS dysregulation results in renal organ damage.Citation18 The RAS is one of the pathophysiologic factors for the development of nephropathies.Citation19 In this sense, RAS is involved in the development of HSP/HSPN. Second, angiotensin II is main biologically product of RAS, AGT interacted with renin to produce angiotensin I, which was then catalyzed to form angiotensin II. Hence, AGT might be associated with the pathogenesis of HSP/HSPN. Finally, gene polymorphism of AGT might affect the AGT concentration. For example, AGT M235T gene polymorphism was closely associated with plasma AGT level, M235T TT genotype carriers have been shown to have 10–20% more plasma AGT level than in M235T MM genotype carriers.Citation20 The above-mentioned evidence indicated that AGT M235T gene polymorphism might be associated with the risk of HSP/HSPN.
Our findings were partially consistent with the above-mentioned mechanisms. However, AGT M235T gene polymorphism was not associated with HSPN risk, which might be due to the facts that AGT regulated the function of RAS indirectly. In addition, the renal damage of HSPN is comparatively mild. The mild renal damage might not be associated with AGT M235T gene polymorphism.
In the past, there were a number of meta-analyses and studies investigating the association between AGT M235T gene polymorphism and immune-related and renal diseases. A meta-analysis by Ding et al.Citation21 reported that AGT M235T gene polymorphism was not associated with diabetic nephropathy. Zhou et al.Citation22 reported that no marked association between AGT M235T gene polymorphism and ESRD was observed in overall populations. Mao et al.Citation23 reported that AGT M235T gene polymorphism was not associated with the risk of IgA nephropathy. Arfa et al.Citation24 reported that AGT M235T gene polymorphism was not associated with the development of hypertension. Eroglu et al.Citation25 reported that there were no differences in the frequencies of the AGT M235T gene polymorphism between Turkish patients with type 2 DM with and without nephropathy. Takakura et al.Citation26 reported that TT genotype of the AGT M235T gene polymorphism was positively related to visceral obesity and hyperinsulinemia in obese Japanese women. Zulian et al.Citation27 reported that AGT M235T gene polymorphism was associated with polycystic ovary syndrome. These previous findings strongly suggest that AGT M235T gene polymorphism might not be associated with renal damage.
Although our study has obvious strengths, such as that the participants were all form Asia, the genotype distribution of control groups were all in HWE, the control group of HSPN cases are all HSP without nephropathy, several limitations should be considered in our meta-analysis. First, the heterogeneities across studies might affect the results, although no evidence of marked publication bias was noted. Second, studies included were only from Asia, which made it difficult to extrapolate our conclusions to other populations. More studies in other regions, such as Europe, should be performed in the future. Finally, the limited number of participants decreases the statistical power.
Taken together, this meta-analysis suggests M allele was associated with lower risk of HSP in adult, TT genotype was associated with the susceptibility to HSP in adult. However, more studies are needed to validate our findings.
Declaration of interest
There is no conflict of interest for all authors. This study was supported by Grants from the National Basic Research Program of China 973 Program (Nos. 2012CB517602 and 2013CB530604), the National Natural Science Foundation of China (Nos. 81170635 and 81270785) and the Research and innovation Project for College Graduates of Jiangsu Province, China (grant number CXLX13_556).
References
- Gonzalez LM, Janniger CK, Schwartz RA. Pediatric Henoch Schönlein purpura. Int J Dermatol. 2009;48:1157–1165
- Bogdanovic R. Henoch Schönlein purpura nephritis in children: Risk factors, prevention and treatment. Acta Paediatr. 2009;98:1882–1889
- Hitomi S, Michiko I, Hiroshi S, Yunosuke O. Risk factors of renal involvement and significant proteinuria in Henoch Schönlein purpura. Eur J Pediatr. 2002;161:196–201
- Raizada V, Skipper B, Luo W, Griffith J. Intracardiac and intrarenal renin-angiotensin systems: Mechanisms of cardiovascular and renal effects. J Investig Med. 2007;55:341–359
- Fuentes Y, Hernández AM, García-Roca P, et al. Urinary MCP-1/creatinine in Henoch-Schönlein purpura and its relationship with nephritis. Pediatr Nephrol. 2014;29:1047–1052
- Anderson PW, Do YS, Hsueh WA. Angiotensin II causes mesangial cell hypertrophy. Hypertension. 1993;21:29–35
- Ruiz-Ortega M, Lorenzo O, Egido J. Angiotensin III up-regulates genes involved in kidney damage in mesangial cells and renal interstitial fibroblasts. Kidney Int Suppl. 1998;68:S41–S45
- Oudart N. The renin-angiotensin system: current data. Ann Pharm Fr. 2005;63:144–153
- Nalbantoglu S, Tabel Y, Mir S, et al. Association between RAS gene polymorphisms (ACE I/D, AGT M235T) and Henoch-Schönlein purpura in a Turkish population. Dis Markers. 2013;34:23–32
- Desong Liu, Fang Lu, Songhui Zhai, et al. Renin-angiotensin system gene polymorphisms in children with Henoch-Schönlein purpura in West China. J Renin Angiotensin Aldosterone Syst. 2010;11:248–255
- Ozkaya O, Söylemezoğlu O, Gönen S, et al. Renin-angiotensin system gene polymorphisms: Association with susceptibility to Henoch-Schönlein purpuraand renal involvement. Clin Rheumatol. 2006;25:861–865
- Jindan An, Sufen Guo, Hongtao Zhao, et al. Association between ATG and ACE gene polymorphisms with genetic susceptibility to HSP/HSPN of adult. Int J Genet. 2012;35:258–267
- Chunlei Wu, Zhongmin Fan, Zhengkun Xia, et al. Association of angiotensinogen gene polymorphism with Henoch Schönlein purpura and Henoch-Schönlein purpura nephritis. J Clin Pediatr. 2009;27:314–316
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387–1395
- Lee YH, Choi SJ, Ji JD, et al. Associations between the angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to vasculitis: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2012;13:196–201
- Vianna HR, Soares CM, Tavares MS, et al. Inflammation in chronic kidney disease: The role of cytokines. J Bras Nefrol. 2011;33:351–364
- Boom BW, Mommaas M, Daha MR, Vermeer BJ. Complement-mediated endothelial cell damage in immune complex vasculitis of the skin: Ultrastructural localization of the membrane attack complex. J Invest Dermatol. 1989;93(2 Suppl):68S–72S
- Kagami S. Involvement of glomerular renin-angiotensin system (RAS) activation in the development and progression of glomerular injury. Clin Exp Nephrol. 2012;16:214–220
- Mitchell KD, Botros FT, Navar LG. Intrarenal renin-angiotensin system and counteracting protective mechanisms in angiotensin II-dependent hypertension. Acta Physiol Hung. 2007;94:31–48
- Chen S, Zhang L, Wang HW, et al. The M235T polymorphism in the angiotensinogen gene and heart failure: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2012;15:190–195
- Ding W, Wang F, Fang Q, et al. Association between two genetic polymorphisms of the renin–angiotensin–aldosterone system and diabetic nephropathy: A meta-analysis. Mol Biol Rep. 2012;39:1293–1303
- Zhou TB, Yin SS, Qin YH. Association of angiotensinogen M235T gene polymorphism with end-stage renal disease risk: A meta-analysis. Mol Biol Rep. 2013;40:765–772
- Mao S, Huang S. Association of angiotensinogen gene M235T polymorphism with the risk of IgA nephropathy: A meta-analysis. Ren Fail. 2014;36:466–472
- Arfa I, Nouira S, Abid A, et al. Lack of association between renin-angiotensin system (RAS) polymorphisms and hypertension in Tunisian type 2 diabetics. Tunis Med. 2010;88:38–41
- Eroglu Z, Cetinkalp S, Erdogan M, et al. Association of the angiotensinogen M235T and angiotensin-converting enzyme insertion/deletion genepolymorphisms in Turkish type 2 diabetic patients with and without nephropathy. J Diabetes Complicat. 2008;22:186–190
- Takakura Y, Yoshida T, Yoshioka K, et al. Angiotensinogen gene polymorphism (Met235Thr) influences visceral obesity and insulin resistance in obese Japanese women. Metabolism. 2006;55:819–824
- Zulian E, Sartorato P, Schiavi F, et al. The M235T polymorphism of the angiotensinogen gene in women with polycystic ovary syndrome. Fertil Steril. 2005;84:1520–1521