Abstract
Background/Aim: The prevalence of pulmonary hypertension (PH) is reported between 17 and 56% in hemodialysis (HD) patients. Pathogenesis of PH in HD patients is still unclear. Malnutrition associating impaired pulmonary function tests in HD patients previously reported. Present study aimed to investigate an association between PH and nutrition and inflammation HD patients. Patients/Methods: Total 179 HD patients (109 M, 70 F) were included. Pulmonary artery pressure (PAP) and ejection fraction (EF) percentage was determined by echocardiography after a midweek HD session. Bioimpedance analyses were performed after dialysis. Percent body fat mass truncal fat (%), total body water (%), body-mass index was determined. Serum 25-OH vitamin D, albumin, lipid parameters, C-reactive protein (CRP), calcium, phosphorus, parathyroid hormone, ferritin levels, and hemogram were studied. Results: Pulmonary hypertension (PAP >35 mmHg) was found in 48 (26.8%) of 179 patients studied. Body-mass index (BMI) was negatively correlated with PAP (r = −0.34; p = 0.02). HD vintage, prevalence of diabetes, sex, type of vascular access were not different between patients with PH and without PH. Patients with PH were older (68.1 ± 14.4; 61.3 ± 14.7; p = 0.005). Percent body fat (19.8 ± 8.1% vs. 28.1 ± 10%; p = 0.001), albumin (3.4 ± 0.5 g/dl vs. 3.9 ± 3.3 g/dl; p = 0.0001), truncal fat (16.8 ± 10.7 vs. 26.4 ± 10.5; p = 0.001), triglyceride (147.9 ± 88.5 vs. 182.1 ± 97.7 mg/dl; p = 0.03), and total cholesterol (146.9 ± 34.5 vs. 169.5 ± 43 mg/dl; p = 0.004) levels were significantly lower in patients with PH than with no PH. Logistic regression analysis revealed that increased percent body fat, albumin, and total cholesterol associate with a decreased risk of PH. Conclusion: Present study demonstrated a significant association between malnutrition and PH in HD patients. Those results should be confirmed by further prospective studies including cytokine levels and spirometric measurements.
Introduction
Pulmonary hypertension (PH) is a complex hemodynamic alteration which may result from disparate causes, is described as an increase in mean pulmonary arterial pressure (mPAP ≥25 mmHg). PH in chronic kidney disease (CKD) is classified as a different group (Group 5) which consists of PH with unclear and/or multifactorial mechanisms.Citation1,Citation2 Cardiac catheterization revealed a prevalence of PH in 71% of patients with pre-dialysis CKD and 77% of hemodialysis (HD) patients having dyspnea unexplained by other causes.Citation3 Studies based on echocardiography detected prevalences of PH between 17 and 56% in patients with end-stage renal disease (ESRD).Citation4–9
PH is a newly emerging entity in HD patients. The mortality rate of HD patients with elevated pulmonary artery pressure (PAP) has been found to be significantly higher than those patients with normal PAP.Citation4 Numerous clinical, hemodynamic, and metabolic abnormalities, such as anemia, fluid overload, and increased CO because of AV fistula, oxidative stress, and alterations in the release of vasoactive mediators, such as endothelin-1 (ET-1) and nitric oxide (NO), have been suggested to have a significant role in the pathogenesis of PH in HD patients.Citation4,Citation10–12 A chronic inflammatory state is a prominent feature in ESRD patients who are undergoing maintenance HD.Citation13 A high prevalence of protein energy malnutrition leading to systemic inflammation has been shown in those patients also. Previously Ulubay et al.Citation14 demonstrated a negative correlation between pulmonary functions (FEV1, FVC, FEF25–75%, PEF) and malnutrition inflammation score in HD patients. Another prospective study on maintenance of HD patients revealed lower albumin but similar C-reactive protein (CRP) levels in patients with PH than with no pulmonary hypertension.Citation15
The aim of the present study is to investigate the prevalence of PH and a possible association between pulmonary hypertension and nutritional, biochemical, inflammatory markers, and body composition in long-term HD patients.
Patients and methods
Total of 179 HD patients (109 M, 70 F) who were on HD at least for six months were involved in this study. All patients involved were on regular 4 h thrice weekly regular HD at Baskent University Alanya Hospital Hemodialysis Unit via arteriovenous fistula using same method of cannulation and biocompatible polysulphone high-flux dialyzers (Allmed Polypure 1.8–2 m2, Allmed Medical GmbH, Dresden, Germany). Dialysate flow rates were 500–800 mL/min and blood flow rates were between 250 and 400 mL/min. Of the 179 patients, 51 patients (28.5%) had diabetes and 128 patients (73.7%) had no diabetes at the time of the study. Primary kidney diseases were diabetic nephropathy in 48 (26.8%), hypertension in 49 (27.3%), chronic glomerulonephritis in 29 (16.2%), nephrolithiasis in 9 (5.1%), polycystic kidney disease in 9 (5%), unknown in 19 (10.6%), obstructive nephropathy in 12 (6.7%), amyloidosis in 4 (2.3%).
Exclusion criteria
Patients with known acute or chronic pulmonary disease or known history of smoking, clinical diagnosis of decompensate cardiac failure or measured ejection fraction below 40% before the study, coronary artery disease or by-pass surgery, chronic atrial fibrillation, were excluded. Patients who missed two or more HD sessions in the last one month, abused drugs, body-mass index (BMI) ≥ 40 kg/m2 were excluded. Patients who had a change in dry weight or anti-hypertensive medications within two weeks were also excluded. Patients with uncontrolled hyperphosphatemia, uncontrolled hypertension, adherence problems to diet and medications, known to have active malignant and inflammatory, infectious, or hepatic diseases were excluded. None of the patients were under treatment of steroids, fish-oil, active vitamin D, or derivatives, at the time of the study. None of the patients had a history of bone disease, active bone fracture, diseases causing intestinal malabsorption.
Clinical and demographic data collection
Patient’s history of diabetes, rheumatism, cardiac, or vascular diseases, primary kidney diseases, smoking habits, medications, and duration of HD were recorded at baseline from patient interviews, and HD data sheets. Duration of HD is recorded as months. Demographic characteristics and basal laboratory results of the study group were shown in . Dry body weight is taken as the mean of last three pre-dialysis body weights. Body-mass index is calculated with division of dry body weight (kg) to square of height (m2).
Table 1. Baseline clinical, demographic, and laboratory characteristics of study group.
Biochemical analyses
In all patients blood samples were drawn at a midweek HD session from antecubital vein with standard method. 25-OH (hydroxy) vitamin D levels were studied with chemiluminescent microparticle immunometric assay (Architect I 10/Abbott®, Abbott Laboratories, Abbott Park, IL). Intact PTH was measured by chemiluminescence immunometric assays using Siemens Immulite 2000® (Siemens Healthcare Diagnostics, Deerfield, IL). Calcium, phosphorus, albumin, and CRP tests were measured by standard laboratory methods using the c8000 Architect (Abbott® Laboratories, Abbott Park, IL). Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and plasma triglyceride (TG) concentrations were undertaken using an oxidase-based technique by the Roche/Hitachi® modular system (Mannheim, Germany).
Bio impedance analysis
Basal metabolic rate, muscle mass, total fat mass and percentage of total fat (%), truncal fat mass, percentage of fat at upper and lower extremities separately measured by bio impedance analysis method (Tanita® Body Composition Analyser, BC-418; Tanita Corp., Arlington Heights, IL).
Echocardiography
All patients underwent two-dimensional echocardiography (Sonos® 4500 HP; Hewlett-Packard Company, Palo Alto, CA) with a 2.5 MHz multiphase array probe to assess cardiac valvular calcification after dialysis at their dry body weight during patients lying in left decubitus position. All echocardiographs were performed according to recommendations of the American Society of Echocardiography and were analyzed by a single experienced cardiologist who was blinded to all clinical details.Citation16 In all patients, PAP was obtained by adding the estimated right atrial pressure to the tricuspid regurgitation velocity 2 × 4. The right atrial pressure was estimated as 5 mmHg if there was no engorgement in inferior vena cava (more than 50% difference in diameter by tidal inspiration). It was assumed as 10 mmHg, if there was less than 50% difference in diameter of inferior vena cava. PAP >35 mmHg is defined as pulmonary hypertension which is generally accepted.Citation17–19
Indices of dialysis adequacy
Total weekly urea clearances of all patients were measured using standard methods as described previously.Citation20
Statistical analyses
Statistical analyses were performed by using software SPSS 11.0.1 (April 2002; IBM® Corp., Armonk, NY). Assumption of normal (Gaussian) distribution was tested by the One Sample Kolmogorov-Smirnov test. Simple correlations were performed using Pearson’s or Spearman’s correlation analyses as appropriate. Comparisons of variables between groups were performed Student’s t-test or Mann-Whitney U-tests in accordance with the distribution pattern of variable. Binary logistic regression analysis is used to determine independent effects of variables on pulmonary hypertension.
Results
Baseline demographic characteristics and baseline laboratory results of the study group are summarized in .
Echocardiography revealed pulmonary hypertension (PH: PAP>35 mmHg) in 48 (26.8%) patients.
Simple correlations
Pulmonary artery pressure was negatively correlated with BMI (r = −0.34; p = 0.002), percentage of body fat (r = −0.316; p = 0.004), truncal fat mass (r = −0.34; p = 0.002), pre-dialysis body weight (r = −0.28; p = 0.01), dry (post-dialysis) body weight (r = −0.3; p = 0.004), triglyceride (r = −0.23; p = 0.005), total cholesterol (r = −0.26; p = 0.002) and albumin (r = −0.23; p = 0.0001) (). PAP had no correlation with CRP, hemoglobin, dialysis vintage, and Kt/v.
C-reactive protein levels was positively correlated with age (r = 0.29; p = 0.0001), negatively correlated with albumin (r = −0.465; p = 0.0001), hemoglobin (r = −0.31; p = 0.001) and 25-hiydroxy vitamin D levels (r = −0.221, p = 0.02).
Albumin had positive correlations with triglyceride (r = 0.3; p = 0.0001), hemoglobin (r = 0.23; p = 0.01). Dialysis vintage had a negative correlation with total muscle mass (r = −0.27; p = 0.02).
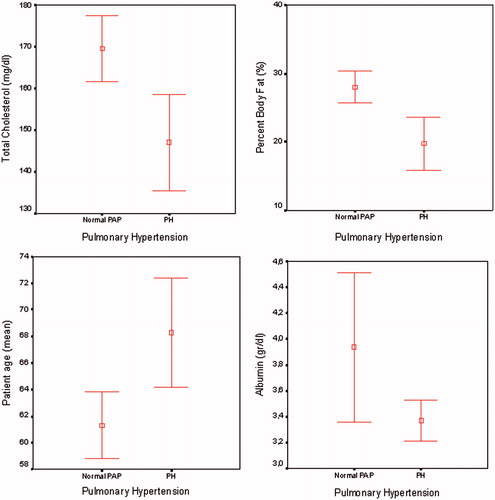
Comparison of the body composition, nutritional, inflammatory, and metabolic parameters between HD patients having PH and with no pulmonary hypertension revealed that patients with PH were older (68.3 ± 14.4 vs. 61.3 ± 14.7; p = 0.005), had lower BMI (25.1 ± 3.9 vs. 27.7 ± 5.3; p = 0.048), lower percentage of body fat (19.8 ± 8.1% vs. 28.1 ± 10%; p = 0.001), lower truncal fat mass (16.8 ± 10.7 vs. 26.4 ± 10.5; p = 0.001), lower albumin (3.4 ± 0.5 g/dL vs. 3.9 ± 3.3 g/dL; p = 0.0001), lower triglyceride (147.9 ± 88.5 vs. 182.1 ± 97.7 mg/dL; p = 0.03) and total cholesterol (146.9 ± 34.5 vs. 169.5 ± 43 mg/dL; p = 0.004) (). C-reactive protein, EF, HD vintage, Kt/v urea and other metabolic parameters were not different between patients with PH and with no PH ().
Table 2. Clinical, laboratory, and bioimpedance data in groups of HD patients with and without PH.
A binary logistic regression analysis model, in which PH is defined as dependent variable, age, body fat percent, albumin, triglyceride, and total cholesterol levels as independent variables, revealed that increased body fat percent, albumin, and total cholesterol levels were associated with a decreased risk of pulmonary hypertension ().
Table 3. Binary logistic regression analysis model; effects of different parameters on dependent variable pulmonary hypertension.
Discussion
Present study revealed a high prevalence (26.8%) of PH in HD patients that was in similar range (17–56%) previously reported in literature.Citation4–9 Patients with PH were older than patients with normal PAP (68.3 ± 14.4 vs. 61.3 ± 14.7; p = 0.005). In accordance with our findings age related increase in PAP is shown in normal population and in HD patients previously.Citation21,Citation22
Patients with PH had lower BMI, lower percentage of body fat and truncal fat mass, albumin, triglyceride, and total cholesterol levels than patients with normal PAP in present study. On the other side, CRP levels were similar between two groups. Those findings suggest a relationship between wasting and PH in HD patients independent of inflammation. To our knowledge, there is barely any previous report that demonstrates an association between nutritional parameters and pulmonary hypertension. Nevertheless Nascimento et al.Citation23 reported a close relationship between lung function, inflammation, and malnutrition in patients with CKD. They suggest that malnutrition and inflammation are associated with a loss of muscle mass and that may also affect respiratory muscles, potentially contributing to impaired pulmonary function in patients with CKD. Their findings are supported by Yoon et al.Citation24 with a similar investigation in 106 HD patients. They showed that malnourished HD patients had higher CRP levels, older age, lower forced vital capacity (FVC), and forced expiratory volume 1 (FEV1) in spirometry than normal patients on HD. Relationship between pulmonary functions (spirometry) and pulmonary hypertension in HD patients is not studied previously. Although Grossman et al.Citation25 could not demonstrate a correlation between spirometry and PAP in young healthy subjects, a possible association between malnutrition, pulmonary functions, and PAP in HD patients is needed to be further investigated.
Figure 1. Correlations between PAP (mmHg) and albumin (g/dL), total cholesterol (mg/dL) levels and body fat percent (%).
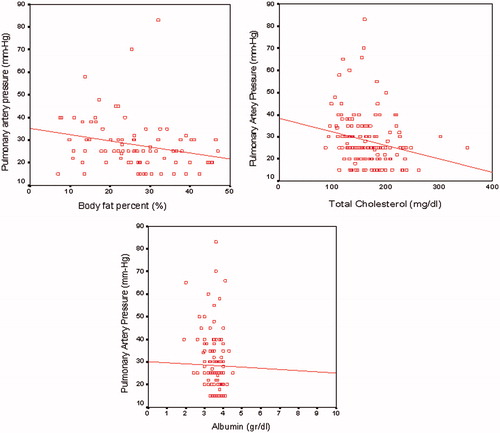
Figure 2. Comparison of age, body fat percent (%), albumin (g/dL), and total cholesterol (mg/dL) levels between patients with and without PH.
Present data suggests a correlation between malnutrition and PH in HD patients. It is well known that many patients with ESRD have protein-calorie malnutrition, with the reported frequency ranging from 23 to 73%.Citation26,Citation27 Stenvinkel et al.Citation28 described different types of malnutrition in uremic patients. One of that malnutrition associated with inflammation (type 2) and second non-inflammatory malnutrition (type 1), which associates with low intake of protein and energy due to uremic anorexia, under dialysis and physical inactivity. In the present study, HD patients having PH had similar CRP levels with patients having normal PAP. Therefore, they could be classified as type 1 malnourished group. Yoo et al.Citation15 reported a higher mortality rate in HD patients with PH who also had low albumin levels than patients with PH and normal albumin levels. They showed lower albumin levels in patients with PH than having normal PAP and suggested that hemodilution as the presumable mechanism to explain the propensity to having lower albumin level in PH group. However, in the present study, combined lower levels of albumin, triglyceride, and cholesterol levels and lower BMI in the PH group suggests malnutrition rather than hemodilution should be underlying mechanism of hypoalbuminemia. In concordance with our results, Mousavi et al.Citation29 demonstrated an association between low serum albumin, hemoglobin levels, and PH in HD patients. As difference in hemoglobin levels was similar between patients with and without PH in our study, Akmal et al.Citation30 proved that increased parathyroid hormone (PTH) levels in CKD could cause calcification of pulmonary artery and leading to PH. We could not detect a difference in parameters, PTH, calcium, phosphorus, Ca × P product and 25-OH vitamin D levels between patients having PH and normal PAP. All patients involved in the present study were undergoing HD via active brachiocephalic arteriovenous fistulas at the time of the study. Previously blood flow of the fistula is suggested to be a major cause of PH in some studies.Citation8,Citation31,Citation32 Yigla and co-workersCitation9 proved that an increase in the flow of the fistula is positively correlated with an increase in EF and subsequently increase in PAP. Havlucu et al.Citation5 demonstrated that HD patients with PH had high EF and they attributed this finding to increase in blood flow of fistula, anemia, and secondary hypervolemia due to CKD. In contrast to those findings, Fadaii et al.Citation22 detected a lower EF in HD patients with higher PAP. Although we did not measure the flow rate of the fistulas, EF was not different between patients with and without PH.
As an inverse perspective, pulmonary hypertension itself could be reason for cachexia and malnutrition. In patients with primary PH development of cachexia is shown and linked to an increased production of putative satiety hormone (PYY-36).Citation33 The gut hormone PYY-36 acts as a terminator of hunger, reduces appetite, and 24 h food intake by binding to neuropeptide Y, Y2 receptor in the hypothalamic arcuate nucleus.Citation34 A recent investigation revealed a progressive decrease in body weight after experimentally induced PH in mice.Citation35 Those evidences suggest that wasting might be a result of PH rather than being a cause. On the other hand, cytokines may have a major role in the development of both PH and wasting at the same time. A recent investigation in patients with heart failure revealed a direct correlation between cytokines (TNF-α, IL-6, hsCRP, and NT-proBNP) and PAP.Citation36 Findings of present study is not enough to make a comment about the direction of cause-effect relationship between wasting and PH; however, those results are first to draw attention to the existence of such a correlation between PH and malnutrition independent of inflammation.
In conclusion, the present study demonstrated first time a negative association between nutritional parameters and PH in HD patients. Our study had some limitations, first study design was cross-sectional and so we have no change to make a comment about malnutrition and pulmonary hypertension which was prior? Second, we did not detect the levels of other inflammatory proteins, such as TNF-alpha, IL-6, fibrinogen. Third, we did not perform spirometric analyses and their correlation with PAP. Fourth, our HD patient population is merely old and results could not be generalized for younger patient groups. Further, prospective controlled studies are needed to confirm our results and enlighten the direction of this complex relationship.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT), Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):1219–1263
- Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. Am Coll Cardiol. 2009;54(1 Suppl):43–54
- Pabst S, Hammerstingl C, Hundt F, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: Results of the PEPPER-study. PLoS One. 2012;7(4):35310
- Yigla M, Nakhoul F, Sabag A, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123(5):1577–1582
- Havlucu Y, Kursat S, Ekmekci C, et al. Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74(5):503–510
- Bozbas SS, Akcay S, Altin C, et al. Pulmonary hypertension in patients with end-stage renal disease undergoing renal transplantation. Transplant Proc. 2009;41(7):2753–2756
- Ramasubbu K, Deswal A, Herdejurgen C, Aguilar D, Frost AE. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: Prevalence and clinical significance. Int J Gen Med. 2010;3:279–286
- Abdelwhab S, Elshinnawy S. Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28(6):990–997
- Yigla M, Fruchter O, Aharonson D, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75(9):969–975
- Nakhoul F, Yigla M, Gilman R, Reisner SA, Abassi Z. The pathogenesis of pulmonary hypertension in hemodialysis patients via arterio-venous access. Nephrol Dial Transplant. 2005;20(8):1686–1692
- Amin M, Fawzy A, Hamid MA, Elhendy A. Pulmonary hypertension in patients with chronic renal failure: Role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124(6):2093–2097
- Ottosson-Seeberger A, Ahlborg G, Hemsén A, Lundberg JM, Alvestrand A. Hemodynamic effects of endothelin-1 and big endothelin-1 in chronic hemodialysis patients. J Am Soc Nephrol. 1999;10(5):1037–1044
- Ho LC, Wang HH, Peng YS, et al. Clinical utility of malnutrition-inflammation score in maintenance hemodialysis patients: Focus on identifying the best cut-off point. Am J Nephrol. 2008;28(5):840–846
- Ulubay G, Kupeli E, Karakan S, et al. Association between “Malnutrition Inflammation Score” and airway obstruction in patients awaiting renal transplantation: A preliminary study. Transplant Proc. 2011;43(2):441–444
- Yoo HH, Martin LC, Kochi AC, et al. Could albumin level explain the higher mortality in hemodialysis patients with pulmonary hypertension? BMC Nephrol. 2012;13:80
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367
- Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart. 2011;97(8):612–622
- Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(Suppl 1):S55–S66
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):40–47
- Wang AY, Woo J, Lam CW, et al. Is a single time point C-reactive protein predictive of outcome in peritoneal dialysis patients? J Am Soc Nephrol. 2003;14(7):1871–1879
- Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119(20):2663–2670
- Fadaii A, Koohi-Kamali H, Bagheri B, Hamidimanii F, Taherkhanchi B. Prevalence of pulmonary hypertension in patients undergoing hemodialysis. Iran J Kidney Dis. 2013;7(1):60–63
- Nascimento MM, Qureshi AR, Stenvinkel P, et al. Malnutrition and inflammation are associated with impaired pulmonary function in patients with chronic kidney disease. Nephrol Dial Transplant. 2004;19(7):1823–1828
- Yoon SH, Choi NW, Yun SR. Pulmonary dysfunction is possibly a marker of malnutrition and inflammation but not mortality in patients with end-stage renal disease. Nephron Clin Pract. 2009;111(1):1–6
- Grossman A, Benderly M, Prokupetz A, Gordon B, Kalter-Leibovici O. Correlation between spirometry values and pulmonary artery pressure in young healthy subjects. Respir Care. 2014;59(3):371–374
- Cianciaruso B, Brunori G, Kopple JD, et al. Cross-sectional comparison of malnutrition in continuous ambulatory peritoneal dialysis and hemodialysis patients. Am J Kidney Dis. 1995;26(3):475–486
- Leavey SF, Strawderman RL, Jones CA, Port FK, Held PJ. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31(6):997–1006
- Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. 2000;15(7):953–960
- Mousavi SA, Mahdavi-Mazdeh M, Yahyazadeh H, et al. Pulmonary hypertension and predisposing factors in patients receiving hemodialysis. Iran J Kidney Dis. 2008;2(1):29–33
- Akmal M, Barndt RR, Ansari AN, Mohler JG, Massry SG. Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int. 1995;47(1):158–163
- Dagli CE, Sayarlioglu H, Dogan E, et al. Prevalence of and factors affecting pulmonary hypertension in hemodialysis patients. Respiration. 2009;78(4):411–415
- Amin M, Fawzy A, Hamid MA, Elhendy A. Pulmonary hypertension in patients with chronic renal failure: Role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124(6):2093–2097
- le Roux CW, Ghatei MA, Gibbs JS, Bloom SR. The putative satiety hormone PYY is raised in cardiac cachexia associated with primary pulmonary hypertension. Heart. 2005;91(2):241–242
- Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654
- Ahn B, Empinado HM, Al-Rajhi M, Judge AR, Ferreira LF. Diaphragm atrophy and contractile dysfunction in a murine model of pulmonary hypertension. PLoS One. 2013;8(4):e62702
- Dolenc J, Šebeštjen M, Vrtovec B, Koželj M, Haddad F. Pulmonary hypertension in patients with advanced heart failure is associated with increased levels of interleukin-6. Biomarkers. 2014;19(5):385–390

