Abstract
Acute renal failure (ARF) is one of the most common problems encountered in hospitalized critically ill patients. In recent years great effort has been focused on the introduction of herbal medicine as a novel therapeutic agent for prevention of ARF. Hence, the current study was designed to investigate the effect of Açai berry extract (ABE) on glycerol-induced ARF in rats. Results of the present study showed that rat groups that received oral ABE in a dose of 100 and 200 mg/kg/day for 7 days before or 7 days after induction of ARF by a single intramuscular glycerol injection reported a significant improvement in kidney functions tests [decrease in serum urea, serum creatinine, and blood urea nitrogen (BUN)] when compared to the ARF model groups. Moreover, there was significant amelioration in renal oxidative stress markers [renal catalase (CAT), renal reduced glutathione (GSH)] and renal histopathological changes in the ABE-treated groups when compared to ARF model groups. The most significant improvement was reported in the groups where ABE was administered in a dose 200 mg/kg/day. These results indicate that ABE has a potential role in ameliorating renal damage involved in ARF.
Introduction
Acute renal failure (ARF) is a critical clinical situation that is usually associated with high mortality rate. Studies reported that rhabdomyolysis-induced myoglobinuric ARF accounts for about 10–40% of all cases of ARF.Citation1
Glycerol-induced ARF is one of the most commonly used models of experimental ARF in rats and is considered as an experimental analogue of human myoglobinuric ARF that results from transfusion accidents or crush injury.Citation2 Myoglobinuric ARF is a uremic syndrome accompanied by skeletal muscle breakdown and intracellular elements that are released into the bloodstream.Citation3
The pathogenesis of glycerol-induced ARF is still not completely understood, but it is generally believed that it may involve oxidative stress and decreased renal blood flow.Citation4,Citation5 Oxidative stress occurs as a result of iron released from heme group of myoglobin. Iron induces the formation of reactive oxygen species that leads to lipid peroxidation and cellular death.Citation6,Citation7
Recent studies have demonstrated that therapeutic interventions can ameliorate glycerol-induced ARF.Citation8,Citation9 And in order to prevent toxic effects of commonly used chemical therapeutic agents, great effort has been focused in recent years on traditional and herbal medicine to provide a novel therapeutic agent for ARF.
Açai (the fruit of Euterpe oleracea Martius palm) has been reported to have the highest antioxidant capacity among all fruits reported to date in the literature. Freeze dried Açai or Açai berry extract (ABE) is a mixture of Açai pulp and skin and has been used in many pharmacological researches to explore the potential therapeutic effect of Açai.Citation10 Studies have shown that addition of Açai pulp to diet improved oxidative stress biomarkers in hypercholesterolemic rats and protected against hydrogen peroxide-mediated damage to lipids and proteins in the cerebral cortex, hippocampus and cerebellum of rats.Citation11,Citation12
To the best of our knowledge, there have been no studies in the literature investigating the effect of ABE on ARF. Accordingly, the purpose of this research was to explore the effect of oral administration of ABE on glycerol-induced ARF in rats.
Methods and materials
Experimental animals
The present study was conducted on 80 male Wistar albino rats weighing from 150 to 200 g. The rats were obtained from the Animal House of the Faculty of Medicine, Alexandria University.
They were housed under optimal laboratory conditions (relative humidity 85 ± 2%, temperature 22 ± 1 °C and 12 h light and 12 h dark cycle).
All experiments were performed in accordance with national animal care guidelines and were preapproved by the Faculty of Medicine, Alexandria University Ethics Committee.
Induction of acute renal failure in rats
Renal failure was induced in rats by a single i.m. injection of 50% (vol/vol) glycerol (Sigma, Germany) diluted in normal saline (10 mL/kg of body weight).Citation13
Animal grouping
Rats were divided into eight equal groups consisting of 10 rats each.
Groups where ABE was administered as prophylaxis for ARF:
Group Pr1: (Normal control group P) rats received 1 mL 2% gum acacia as a vehicle orally every day for 7 days then an IM saline injection was given.
Group Pr2: (ARF model group P) rats received 1 mL 2% gum acacia as a vehicle orally every day for 7 days then induction of ARF was performed.
Group Pr3: Rats received ABE at a dose of 100 mg/kg daily orally for 7 days then induction of ARF was performed. ABE was purchased in a freeze dried form of organic Açai palm berry containing fruit skin and pulp from NOW FOODS, USA.
Group Pr4: Rats received ABE at a dose of 100 mg/kg orally every day for 7 days then induction of ARF was performed.
Groups where ABE was administered as therapy for ARF:
Group T1: (Normal control group T) an IM saline injection was given then rats received 1 mL 2% gum acacia as a vehicle orally every day for 7 days.
Group T2: (ARF model group T) induction of ARF was performed then rats received 1 mL 2% gum acacia as a vehicle orally every day for 7 days.
Group T3: Induction of ARF was performed then rats received ABE at a dose of 100 mg/kg daily orally for 7 days.
Group T4: Induction of ARF was performed then rats received ABE at a dose of 100 mg/kg orally every day for 7 days.
At the end of the experiment (24 h of induction of ARF in prophylactic groups and 7 days after induction of ARF in therapeutic groups), all rats were anaesthetized with sodium pentobarbital (120 mg/kg intraperitoneally). Blood was withdrawn from the heart for estimation of kidney function tests: serum urea, creatinine and blood urea nitrogen (BUN). Rats were then sacrificed using high dose of sodium pentobarbital and both kidneys from rats were isolated. Then kidneys were divided equally into two longitudinal sections. One half of each kidney was placed in formaldehyde solution for histopathological examination by light microscopy. The other half of the kidney was placed into liquid nitrogen and stored at −20 °C until assayed for renal catalase (CAT) and renal reduced glutathione (GSH).
Estimation of renal functions tests
Determination of serum urea, creatinine and BUN were performed using commercial available kits.
Estimation of oxidative stress biomarkers in renal homogenate
One longitudinal half of each kidney was homogenized in phosphate buffer saline (PBS) 50 mM pH (7.4) for estimation of renal CAT and renal GSH.
Estimation of GSH in renal homogenate was done according to Teietz method which is based on the reduction of 5,5′-dithiobis(2-nitrobenzoic acid) to 5-thio-2-nitrobenzoic acid with GSH to produce a yellow compound.Citation14 The reduced chromogen (5-thio-2-nitrobenzoic acid) is directly proportional to GSH concentration and its absorbance was measured at 405 nm by using a commercial kit (Biodiagnostic, Egypt).
Renal CAT was assayed by the method of Sinha which was based on formation of chromic acetate from dichromate and glacial acetic acid in the presence hydrogen peroxide (H2O2). Chromic acetate that produced was measured colorimetrically at 570 nm. One renal catalase unit was defined as the amount of renal catalase which catalyzed the oxidation of 1 μmole H2O2 per minute under assay conditions.Citation15
Renal histopathology
Longitudinal half of each kidney was fixed in a 10% neutral buffered formalin solution, embedded in paraffin, and used for histopathological examination. Five-micrometer thick sections were cut, deparaffinized, and hydrated. For light microscopic purpose, paraffin sections were stained with hematoxylin and eosin (H&E). The sections were examined by a pathologist who was unaware of the treatment which rats had received. The degree of renal damage was assessed according to the following scoring system.Citation16
(a) Necrosis:
Score 1: occasional necrotic tubular cells
Score 2: occasional necrotic tubules
Score 3: 25% cortical tubules necrotic
Score 4: 33% cortical tubules necrotic
Score 5: 50% cortical tubules necrotic
(b) Damaged tubules and casts:
Score 1: occasional damaged tubules
Score 2: occasional damaged tubules and some casts
Score 3: 30% of tubules with casts
Score 4: 50% of tubules with casts
Score 5: 51–100% tubules with casts
The two scores for each kidney were added to give the total damage score (maximum 10).
Statistical analysis
Data were expressed as the mean ± standard deviation for continuous variables. Statistical comparisons between all studied groups were performed using a one way analysis of variance test (ANOVA) while Turkey’s Multiple Comparison Test was used as a post-test to detect significance between all groups by comparing group means. For all analyses, a p < 0.05 was considered statistically significant. Statistical analysis was performed using Graph Pad Prism version 6.0 software (La Jolla, CA).
Results
Effect of Açai berry extract on normal kidney functions and morphology
To exclude the possibility that ABE may affect normal kidney functions, the present study evaluated the effect of oral administration of 100 and 200 mg/kg/day of ABE for 7 days on all assessed parameters included in the present study in groups. The results of those experiments showed that there was no significant change in kidney functions tests, renal oxidative stress parameters and renal histopathology scoring in ABE-treated groups when compared to normal control groups.
Induction of ARF caused significant changes in kidney functions
Induction of ARF by glycerol injection in rats resulted in statistically significant increase in kidney function tests in model groups when compared to normal control groups: there was a significant increase in serum urea (p < 0.001), serum creatinine (p < 0.001) and BUN (p < 0.001) ( and , and ).
Figure 1. Comparison between group Pr1 (normal control group), group Pr2 (ARF model group), group Pr3 (ABE 100 mg/kg/day/7day) and group Pr4 (ABE 200 mg/kg/day for 7 days) regarding kidney function tests when assessed 24 h after induction of ARF. Notes: The statistical significance between the treated groups (Pr3, Pr4), Pr1 normal control group and Pr2 model group was determined using Tukey’s test. *p < 0.001 versus Pr1 normal control group, ∞p < 0.001 versus Pr2 ARF model group. #p < 0.001 versus Pr3 ABE (100 mg/kg/day)-treated group. ABE: Açai berry extract, ARF: acute renal failure, BUN: blood urea nitrogen.
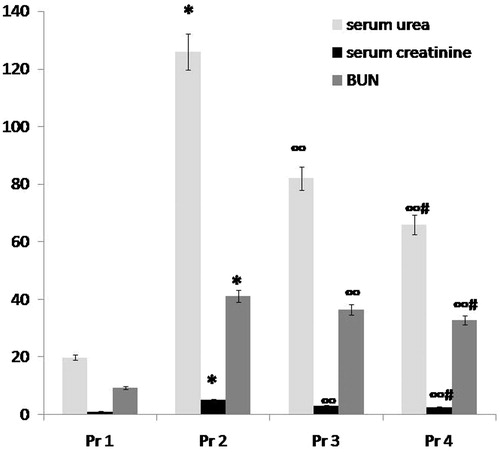
Figure 2. Comparison between group T1 (normal control group), group T2 (ARF model group), group T3 (ABE 100 mg/kg/day for 7 days) and group T4 (ABE 100 mg/kg/day for 7days) regarding kidney function tests when assessed 7 days after induction of ARF. Notes: The statistical significance between T the treated groups (T3, T4), T1 normal control group and T2 model group was determined using Tukey’s test. *p < 0.001 versus T1 normal control group, ∞p < 0.001 versus T2 ARF model group, #p < 0.001 versus T3 ABE (100 mg/kg/day)-treated group. T: treatment, ABE: Açai berry extract, ARF: acute renal failure, BUN: blood urea nitrogen.
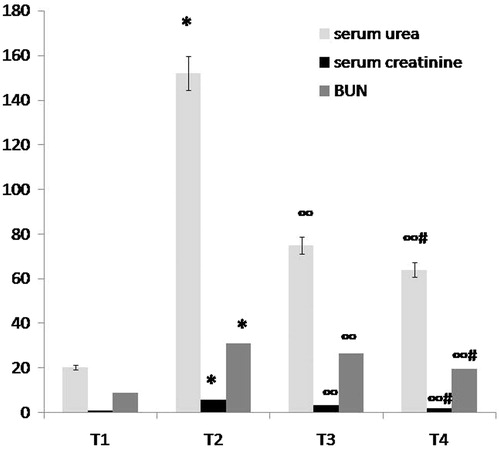
Table 1. Comparison between group Pr1 (normal control group), group Pr2 (ARF model group), group T3 (ABE 100 mg/kg/day for 7days), group Pr3 (ABE 200 mg/kg/day) and group Pr4 (ABE 200 mg/kg/day for 7days), before induction of ARF regarding kidney function tests, renal GSH and renal CAT when assessed 24 h after induction of ARF.
Table 2. Comparison between group T1 (normal control group), group T2 (ARF model group), group T3 (ABE 100 mg/kg/day for 7 days) and group T4 (ABE 200 mg/kg/day for 7 days) regarding kidney function tests, renal GSH and renal CAT when assessed 7 days after induction of ARF.
ABE prevented deterioration of kidney functions in glycerol-induced ARF
Administration of ABE for 7 days in a dose of 100 mg/kg/day in group 5 or in a dose of 200 mg/kg/day in either prophylactic or therapeutic groups resulted in statistically significant improvement in kidney function tests when compared to either model groups (ARF model group P or ARF model group T): there was an significant decrease in serum urea (p < 0.001), serum creatinine (p < 0.001) and BUN (p < 0.001) ( and , and ).
When comparing both drug groups, more significant improvement in kidney function tests was observed in the groups that received ABE in a dose of 200 mg/kg/day (p < 0.001) ( and , and ).
Induction of glycerol-induced ARF caused significant deterioration in renal oxidative stress markers
A significant deterioration in renal oxidative stress markers was detected in ARF model groups when compared to normal control groups: there was significant decrease in renal CAT (p < 0.001) and renal GSH (p < 0.001) ( and , and ).
Figure 3. Comparison between group Pr1 (normal control group), group Pr2 (ARF model group), group Pr3 (ABE 100 mg/kg/day for 7 days) and group Pr4 (ABE 200 mg/kg/day for 7 days) regarding oxidative stress markers (renal GSH and renal CAT) when assessed 24 h after induction of ARF. Notes: The statistical significance between the treated groups (Pr3, Pr4), Pr1 normal control group and Pr2 model group was determined using Tukey’s test. *p < 0.001 versus Pr1 normal control group, ∞p < 0.001 versus Pr2 ARF model group, #p < 0.001 versus Pr3 ABE (100 mg/kg/day)-treated group. Pr: prophylaxis, ABE: Açai berry extract; ARF: acute renal failure; GSH: reduced glutathione.
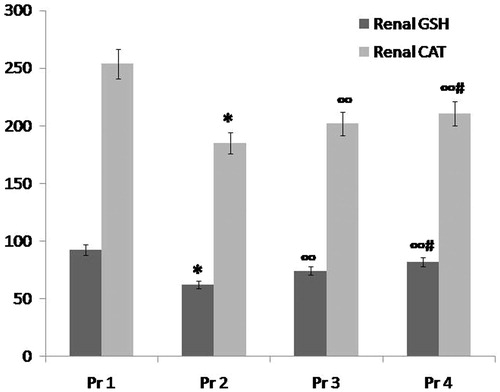
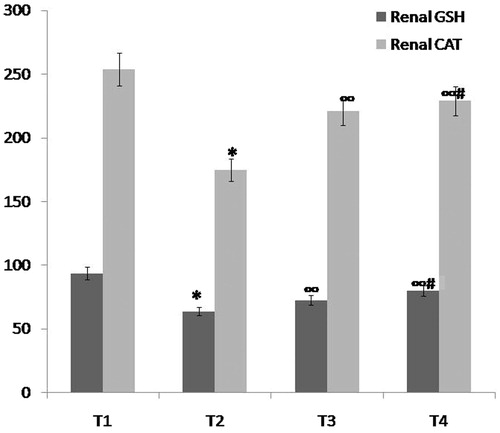
Figure 4. Comparison between group T1 (normal control group), group T2 (ARF model group), group T3 (ABE 100 mg/kg/day for 7 days) and group T4 (ABE 100 mg/kg/day for 7 days) regarding oxidative stress markers (renal GSH and renal CAT) when assessed 7 days after induction of ARF. Notes: The statistical significance between T the treated groups (T3, T4), T1 normal control group and T2 model group was determined using Tukey’s test. *p < 0.001 versus T1 normal control group, ∞p < 0.001 versus T2 ARF model group, #p < 0.001 versus T3 ABE (100 mg/kg/day)-treated group. T: treatment, ABE: Açai berry extract, ARF: acute renal failure, GSH: reduced glutathione.
Antioxidant effect of ABE
Administration of ABE for 7 days in a dose of 100 mg/kg/day and in a dose 200 mg/kg/day resulted in statistically significant improvement in renal oxidative stress markers in both prophylactic and therapeutic groups, respectively when compared to ARF model group P and ARF model group T, respectively: there was significant increase in both renal CAT (p < 0.001) and renal GSH (p < 0.001) ( and , and ).
When comparing both drug groups, more significant improvement in renal oxidative stress markers was observed in the 200 mg/kg/day ABE-treated groups (p < 0.001) (, and ).
Induction of glycerol-induced ARF showed significant renal histopathological changes
Renal sections of rats in the ARF model groups (, , , and ) showed significant renal histopathological damage when compared to the normal control groups (, , , and ) (p < 0.001) ( and ).
Figure 5. Histopathological changes in the prophylactic study groups. (a) Pr1: Normal control group showing a section of normal kidney (H&E staining; 400×). (b) Pr2: ARF model group showing about 50% necrotic cortical tubules with casts (H&E; 400×). (c) Pr3: ABE (100 mg/kg/day)-treated group showing less than 30% necrotic tubules with some casts (H&E; 400×). (d) Pr4: ABE (200 mg/kg/day)-treated group showing occasional necrotic tubules (H&E; 400×).
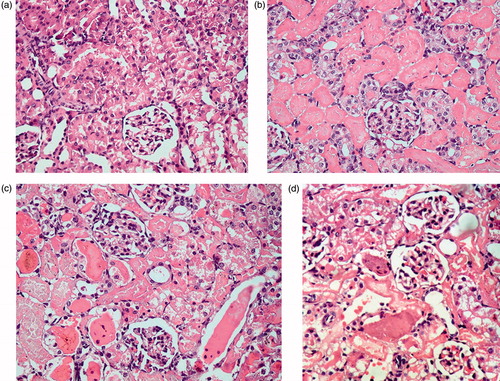
Figure 6. Comparison between group Pr2 (ARF model group), group Pr3 (ABE 100 mg/kg/day for7 days) and group Pr4 (ABE 200 mg/kg/day for 7days) regarding renal histopathological scoring when assessed 24 h after induction of ARF. Notes: The statistical significance between the Pr-treated groups (Pr3, Pr4) and Pr2 model group, was determined using Tukey’s test. ∞p < 0.001 versus Pr2 ARF model group, #p < 0.001 versus Pr3 ABE (100 mg/kg/day)-treated group. Pr: Prophylaxis, ABE: Açai berry extract, ARF: acute renal failure.
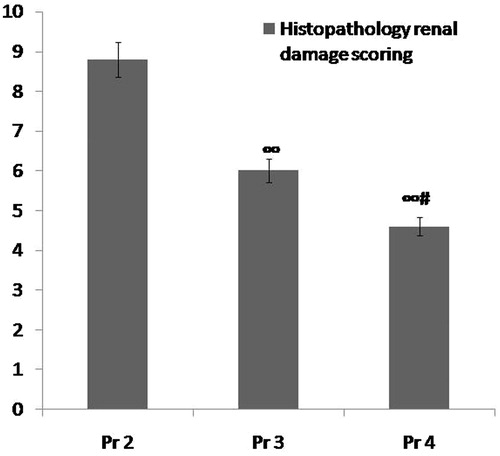
Figure 7. Histopathological changes in the therapeutic study groups. (a) T1: Normal control group showing a section of normal kidney (H&E staining; 400×). (b) T2 ARF model group showing more than 70% necrotic cortical tubules with many casts (H&E; 400×). (c) T3 ABE (100 mg/kg/day)-treated group showing about 30% necrotic tubules with casts (H&E; 400×). (d) T4 ABE (200 mg/kg/day)-treated group showing about some necrotic tubules (H&E; 400×).
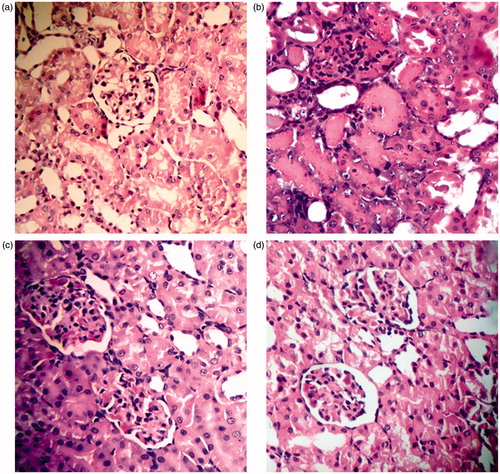
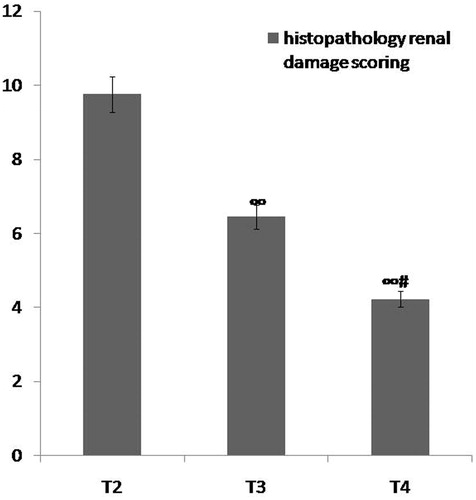
Figure 8. Comparison between group T2 (ARF model group), group T3 (ABE 100 mg/kg/day for 7 days) and group T4 (ABE 100 mg/kg/day for 7 days) regarding renal histopathological scoring when assessed 7 days after induction of ARF. Notes: The statistical significance between the treated groups (T3, T4) and T2 model group was determined using Tukey's test. ∞p < 0.001 versus T2 ARF model group, #p < 0.001 versus T3 ABE (100 mg/kg/day)-treated group. T: treatment, ABE: Açai berry extract, ARF: acute renal failure.
ABE administration caused regression of renal histopathological changes
Significant regression of histopathological changes was recorded in both the ABE prophylactic groups (, , , and ) and treated groups when compared to the ARF model groups (, , , and ) (p < 0.001) ( and ).
When comparing both drug groups, more significant improvement in renal histopathological scoring was observed in the 200 mg/kg/day ABE-treated groups (p < 0.001) ( and , ,,, and ).
Discussion
In the present study, oral administration of ABE resulted in improvement of kidney function tests, renal oxidative stress markers as well as renal pathological changes in rats with glycerol-induced ARF.
Glycerol-induced ARF in rats is characterized by the occurrence of rhabdomyolysis and acute tubular necrosis leading to deterioration of renal functions and hence failure of the kidneys to excrete waste products and to maintain fluid and electrolyte homeostasis.Citation17,Citation18 This is in accordance with the present study which showed significant decline in the renal functions (increase in serum urea, serum creatinine and BUN) in ARF model groups when compared to the normal control groups. Moreover, Xing et al. and Liu et al. reported similar results.Citation19,Citation20
Many therapeutic agents have been recently used as an attempt to prevent renal damage-induced glycerol, e.g. theophylline,Citation16 ferulic acidCitation21 and montelukast.Citation22 However, due to associated adverse effects of such therapeutic agents, natural products like herbs, fruits have been recently introduced to prevent glycerol-induced ARF, e.g. Punica granatum.Citation23
Açai, a well known fruit from the Amazon River floodplain, has proven to contain numerous phytochemicals that possess antioxidant, anti-inflammatory, and anticancerous properties.Citation24
In the literature, this is the first study to explore the effect of ABE on glycerol induced-ARF in rats.
The present study demonstrated that oral administration of ABE protected against glycerol-induced impairment of renal functions through producing significant decrease in serum urea, serum creatinine and BUN when compared to the ARF model groups.
The present study hypothesizes that the possible explanation for improvement in renal function following administration of ABE may be due to its proven role in reduction of oxidative stress and renal histopathological changes.
Oxidative stress plays an important role in the pathogenesis of glycerol-induced ARF. This could be explained in the context that the kidney is known to be an organ with high susceptibility to damage by reactive oxygen species, probably due to the abundance of long chain polyunsaturated fatty acids in the composition of renal lipids.Citation25–28 Similarly, the present study showed significant deterioration of renal oxidative stress markers (decrease in renal CAT and renal GSH) in the ARF model groups when compared to the normal control groups. Manikandan et al.Citation21 reported a significant decrease in activities of superoxide dismutase, catalase, glutathione peroxidase, glutathione-S-transferase and reduced glutathione in rats with glycerol-induced ARF. In addition, Helmy et al. found a significant reduction of renal catalase and superoxide dismutase activities, 48 h following the induction of rhabdomyolysis by intramuscular glycerol.Citation29
To explore the antioxidant role of ABE in the present study, we examined its effect on two important antioxidant enzymes; renal GSH and renal CAT. Results of the current study showed that oral administration of ABE caused significant increase in both renal GSH and renal CAT when compared to their levels in ARF model groups. In addition, more significant improvement was demonstrated in the group that received ABE in a dose of 200 mg/kg/day. The proven antioxidant effect of ABE on other injuries of the kidneys was previously reported. Da Costa et al. demonstrated increase of CAT, superoxide dismutase and glutathione peroxidase after oral administration of Açai stone extract to renovascular hypertensive rats.Citation30 Also the antioxidant effect of Açai has been demonstrated in other organs like brain,Citation12 lungs,Citation31 liverCitation32 in experimental animals. In addition, consumption of Açai pulp and juice was associated with increased plasma antioxidant capacity in healthy human volunteers.Citation33 The freeze-dried ABE that has been used in the current study contains both the Açai pulp and skin. Studies have previously done to uncover the antioxidant contents of Açai pulp which was fractionated and analyzed by researchers for major anthocyanins and other phenolics contents. Fractions extracted using methanol and ethanol were particularly rich in anthocyanins such as cyanidin, delphinidin, malvidin, pelargonidin, and peonidin, whereas the fraction extracted using acetone was rich in other phenolics such as catechin, ferulic acid, quercetin, resveratrol, and synergic and vanillic acids.Citation34 The overall antioxidant mechanism of action of phenols is thought to be through reducing reactive oxygen species production by neutralizing them or by chelating metal ions.Citation35 Da Silva Santos et al. stated that anthocyanins obtained from Açai reduced oxidative stress by restoring GSH/GSSG ratio and net glutamate uptake, and protecting astrocytic membranes from lipid peroxidation.Citation36
Cellular damage of the proximal convoluted tubules together with necrotic changes in the form of casts that occulted the proximal tubular lumen are important landmarks in the pathogenesis of glycerol-induced ARF.Citation2 It has been postulated that glycerol injection leads to the development of acute tubular necrosis by producing a combination of hemoglobinuria and renal ischemia.Citation22,Citation37 The present study revealed significant histopathological changes in kidney sections obtained from rats in ARF model groups when compared to normal control groups. These changes were mostly in the form of severe tubular necrosis together with cast formation. Similar findings were also reported by other investigators.Citation16,Citation17
The present study showed that oral ABE administration caused significant regression of renal histolopathologic changes when compared to ARF model groups. In accordance with our results, de Bem et al. demonstrated that oral administration of Açai seed extract decreased renal structural changes in adult offspring rats (whose mothers were fed a low-protein (LP) diet during pregnancy) probably through vasodilatation and antioxidant effect.Citation38 Da costa et al. reported that oral administration of Açai stone extract to rats leads to significant amelioration of renal histopathological changes in hypertensive rats. Those changes were in the form of decreased endothelial dysfunction and renal vascular structural changes. Such improvement was attributed according to the researchers to the significant proven antioxidant effect of Açai.Citation30
Conclusion
ABE was found to significantly attenuate glycerol-induced ARF changes in rats through ameliorating renal functions and renal oxidative stress. In the literature, this is the first study highlighting this novel finding. Further studies are needed to elucidate the exact cellular mechanism of its renoprotective effect.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chander V, Chopra K. Molsidomine, a nitric oxide donor and l-arginine protects against rhabdomyolysis induced myoglobinuric acute renal failure. Biochim Biophys Acta. 2005;1723:208–214
- Wang SZ, He H, Han R, et al. The protective effects of cobra venom from naja naja atra on acute and chronic nephropathy. Evid Based Complement Alternat Med. 2013;2013:478049
- Ayvaz S, Aksu B, Kanter M, et al. Preventive effects of hyperbaric oxygen treatment on glycerol-induced myoglobinuric acute renal failure in rats. J Mol Histol. 2012;43(2):161–170
- Newaz M, Yousefipour Z. PPARγ and NAD(P)H oxidase system interaction in glycerol-induced acute renal failure: Role of gp91phox subunit of NAD(P)H oxidase. Ren Fail. 2014;36(4):567–574
- Manikandan R, Beulaja M, Thiagarajan R, et al. Ameliorative effect of ferulic acid against renal injuries mediated by nuclear factor-kappaB during glycerol-induced nephrotoxicity in Wistar rats. Ren Fail. 2014;36(2):154–165
- Polo-Romeo FJ, Fernadez-Funez A, Broseta Viana L, Atienza MP, Sanchez Gascon F. Effect of N-acetylcysteine on antioxidant status in glycerol-induced acute renal failure in rats. Ren Fail. 2004;26:613–618
- Vlahovic P, Cvetkovic T, Savic V, Stefanovic V. Dietary curcumin does not protect kidney in glycerol induced acute renal failure. Food Chem Toxicol. 2007;45:1777–1782
- Homsi E, de Brito SM, Janino P. Silymarin exacerbates p53-mediated tubular apoptosis in glycerol-induced acute kidney injury in rats. Ren Fail. 2010;32:623–632
- Wang YD, Zhang L, Cai GY. Fasudil ameliorates rhabdomyolysis-induced acute kidney injury via inhibition of apoptosis. Ren Fail. 2011;33:811–818
- Schauss AG, Wu X, Prior RL, et al. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleracea Mart. (Açai). J Agric Food Chem. 2006;54(22):8604–8610
- de Souza MO, Silva M, Silva ME, Oliveira RP, Pedrosa ML. Diet supplementation with Açai (Euterpe oleracea Mart.) pulp improves biomarkers of oxidative stress and the serum lipid profile in rats. Nutrition. 2010;26:804–810
- Spada PD, Dani C, Bortolini GV, Funchal C, Henriques JA, Salvador M. Frozen fruit pulp of Euterpe oleraceae Mart. (Açai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J Med Food. 2009;12:1084–1088
- Panjehshahin MR, Munsey TS, Collis MG, Bowmer CJ, Yates MS. Further characterization of the protective effect of 8-cyclopentyl-1,3-dipropylxanthine on glycerol-induced acute renal failure in the rat. J Pharm Pharmacol. 1992;44(2):109–113
- Tietez F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–502
- Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–389
- Bowmer CJ, Collis MG, Yates MS. Effect of the adenosine antagonist 8-phenyltheophylline on glycerol-induced acute renal failure in the rat. Br J Pharmac. 1986;88:205–212
- Gu H, Yang M, Zhao X, Zhao B, Sun X, Gao X. Pretreatment with hydrogen-rich saline reduces the damage caused by glycerol-induced rhabdomyolysis and acute kidney injury in rats. J Surg Res. 2014;188(1):243–249
- Ulusoy S, Ozkan G, Alkanat M, Mungan S, Yuluğ E, Orem A. Perspective on rhabdomyolysis-induced acute kidney injury and new treatment options. Am J Nephrol. 2013;38(5):368–378
- Liu Y, Fu X, Gou L, et al. L-citrulline protects against glycerol-induced acute renal failure in rats. Ren Fail. 2013;35(3):367–373
- Xing R, Liu S, Yu H, et al. Protective effect of sulfated chitosan of C3 sulfation on glycerol-induced acute renal failure in rat kidney. Int J Biol Macromol. 2014;65:383–388
- Manikandan R, Beulaja M, Thiagarajan R, et al. Ameliorative effect of ferulic acid against renal injuries mediated by nuclear factor-kappaB during glycerol-induced nephrotoxicity in Wistar rats. Ren Fail. 2014;36(2):154–165
- Homsi E, Janino P, Amano M, Saraiva Camara NO. Endogenous hepatocyte growth factor attenuates inflammatory response in glycerol-induced acute kidney injury. Am J Nephrol. 2009;29(4):283–291
- Singh AP, Singh AJ, Singh N. Pharmacological investigations of Punica granatum in glycerol-induced acute renal failure in rats. Ind J of Pharmacol. 2011;43(5):551–556
- Liedo P, Carey JR, Ingram DK, Zou S. The interplay among dietary fat, sugar, protein and açai (Euterpe oleracea Mart.) pulp in modulating lifespan and reproduction in a Tephritid fruit fly. Exp Gerontol. 2012;47(7):536–539
- Chander V, Singh D, Chopra K. Attenuation of glycerol-induced acute renal failure in rats by trimetazidine and deferoxamine. Pharmacology. 2003;67(1):41–48
- Fishman AI, Alexander B, Eshghi M, Choudhury M, Konno S. Nephrotoxin-induced renal cell injury involving biochemical alterations and its prevention with antioxidant. J Clin Med Res. 2012;4(2):95–101
- Ustundag S, Sen S, Yalcin O, Ciftci S, Demirkan B, Ture M. L-carnitine ameliorates glycerol-induced myoglobinuric acute renal failure in rats. Ren Fail. 2009;31(2):124–133
- Ozbek E. Induction of oxidative stress in kidney. Int J Nephrol. 2012;Article ID:465897
- Helmy M, El-Gowelli H. Montelukast abrogates rhabdomyolysis-induced acute renal failure via rectifying detrimental changes in antioxidant profile and systemic cytokines and apoptotic factors production. Eur J Pharmacol. 2012;683(1–3):294–300
- Da Costa CA, de Oliveira PR, de Bem GF, et al. Euterpe oleracea Mart.-derived polyphenols prevent endothelial dysfunction and vascular structural changes in renovascular hypertensive rats: Role of oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(12):1199–1209
- Kim YS, Jung H, Zerin T, Song HY. Protein profiling of paraquat-exposed rat lungs following treatment with Açai (Euterpe oleracea Mart.) berry extract. Mol Med Rep. 2013;7(3):881–886
- Guerra JF, Magalhães CL, Costa DC, Silva ME, Pedrosa ML. Dietary Açai modulates ROS production by neutrophils and gene expression of liver antioxidant enzymes in rats. J Clin Biochem Nutr. 2011;49(3):188–194
- Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, et al. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich Açai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem. 2008;56:7796–7802
- Poulose SM, Fisher DR, Larson J, et al. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J Agric Food Chem. 2012;60(4):1084–1093
- Afanas'ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavengingmechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763–1769
- da Silva Santos V, Bisen-Hersh E, Yu Y, et al. Anthocyanin-rich Açaí (Euterpe oleracea Mart.) extract attenuates manganese-induced oxidative stress in rat primary astrocyte cultures. J Toxicol Environ Health A. 2014;77(7):390–404
- Carroll R, Kovacs K, Tapp E. The pathogenesis of glycerol-induced renal tubular necrosis. J Path Bact. 1965;89:573–580
- de Bem GF, da Costa CA, de Oliveira PR, et al. Protective effect of Euterpe oleracea Mart (açaí) extract on programmed changes in the adult rat offspring caused by maternal protein restriction during pregnancy. J Pharm Pharmacol. 2014;66(9):1328–1338

