Abstract
Objective: C4 deficiency is the most commonly inherited immune disorder in human. The present study investigated the characteristics of the IgAN patients with low serum C4 levels. Methods: We performed a prospective observational study. Clinical as well as histopathologic parameters were assessed. A Kaplan–Meier survival analysis was performed concerning the primary outcome defined as the serum creatinine increased 1.5-fold from baseline. The prognostic significances of clinical and histopathologic parameters were determined using Cox proportional hazards models. Results: Five-hundred twelve biopsy proven IgAN cases were available for analysis with a median follow-up of 38.4 months. Ninety-nine cases (19.34%) presented with low C4 levels (LowC4 group) and the other 413 cases did not (NlowC4 group). At the time of renal biopsy, renal injury was lighter in the LowC4 group compared with the NlowC4 group. Renal C4 deposition was significantly decreased while IgM deposition was increased in the LowC4 group. A correlation analysis shows that lower C4 levels were associated with better renal presentations at biopsy. However, the risk of developing the primary outcome was significantly greater in those with low C4 levels. Specifically, during the follow-up period, the risk of developing primary outcome was nearly ten folds higher in those with low C4, compared to those without low C4. Conclusion: There is a high prevalence of low C4 levels in IgAN patients. These patients with low C4 levels exhibited better renal presentations at the time of renal biopsy, whereas might be associated with a poor prognosis.
Introduction
Primary immunoglobulin A nephropathy (IgAN) is the most prevalent glomerular disease worldwide, making up 30–40% of all biopsy proven primary glomerulonephritis. It can often cause end stage renal disease (ESRD), and is a major cause of this condition around the world leading to a large burden of illness.Citation1,Citation2
The complement system is a major part of humoral immunity, and has been proposed to play an essential role in many types of glomerular disease including IgAN as an immunological condition.Citation3,Citation4 Complement component 4 (C4) and 3 (C3), are key parts of the complement system that can be measured clinically. A range of data suggests that C4 and/or C3 levels may be important in IgAN. C4, as a subunit of the C3 and C5 convertases, mediates the activation of the lectin pathway which is involved in the pathogenesis of IgAN.Citation5–10 People with C4 deficiency, which is the most commonly inherited immune deficiency known in humans,Citation11,Citation12 are associated with autoimmune disease including systemic lupus erythematosus,Citation13 and the immune-mediated kidney disease including IgAN.Citation14 A deficiency of specific complement isotypes (C4ACitation15 or C4BCitation16) may also be associated with an increased incidence of IgAN. Finally, the homozygous null C4 phenotype frequency may be increased in IgAN and Henoch-Schönlein purpura nephritis.Citation17
Despite these suggestive data, the C4 in the pathogenesis of IgAN remains unclear, and in particular, the prognostic role of C4 has not been clearly defined. We, therefore, studied a large cohort of IgAN patients to assess the relationship between C4 levels and their clinical and histopathologic characteristics, as well as prognosis.
Methods
Study design
This was a prospective observational study that was conducted after approval by the hospital ethics committee.
Participants’ enrollment
All patients with biopsy proven IgAN at initial renal biopsy and CKD stage 1–4 between January 2008 and May 2010 were included. Previous reports indicated that IgAN with asymptomatic microhematuria may not imply a favorable outcome.Citation18,Citation19 So, in general, all patients with heteromorphic microhematuria (++ or greater on a dipstick) or microhematuria, and/or proteinuria were recommended for renal biopsy. IgAN was defined by a renal biopsy proven dominant or co-dominant IgA deposition in the mesangium on immunofluorescence microscopy. Systemic diseases caused secondary IgAN including Henoch–Schönlein purpura, systemic lupus erythematosus, or chronic hepatic diseases were excluded. The following data were collected at the time of renal biopsy: age, gender, mean arterial pressure (MAP) and laboratory examination. In general, patients with proteinuria more than 1 g/d and/or active pathological injuries(interstitial inflammatory cell filtration, severe mesangial proliferation, cellular crescents) received steroid/immunosuppressor(s) according to some available evidencesCitation20,Citation21 as well as the decision of the individual physician.
IgAN Patients available for follow-up were tracked for every 24 weeks. Data including serum creatinine, 24h proteinuria were collected.
Histopathology
Renal biopsy was processed for the light microcopy and direct immunofluorescence. Tissue for histology was fixed in 10% formalin and embedded in paraffin or fixed in 3% paraformaldehyde and embedded in polycol methacrylate. Two-micron-thick sections were cut from paraffin blocks. The paraffin embedded sections were stained with hematoxylin-eosin (HE), periodic acid schiff (PAS), periodic acid-silver methenamine (PASM) and trichrome. The tissue for immunofluorescence was snap-frozen. Six-micron slides were stained with fluorescein-conjugated (FITC) antibodies specific for human IgG, IgM, IgA, C3, and C4. All biopsy slides were reviewed by a single renal pathologist (XL T) who was blinded to all the clinical outcomes.
Complement and immunoglobulin levels
Included patients were separated into different groups either by serum C4 levels (patients with low serum C4 levels (LowC4 group) or those without low serum C4 levels (NLowC4 group)) or by serum C3 levels (patients with low C3 levels (LowC3 group) or those without low C3 levels (NLowC3 group)). Low complement levels were defined as values less than the lower limit of the normal range (serum C3 less than 79mg/dL, serum C4 less than 16mg/dL).
Patients were further separated into four subgroups: Patients with low C3 but not low C4 (Low C3 only group, LC3 group), patients with low C4 but not low C3 (Low C4 only group, LC4 group), low levels in both C3 and C4 group (BL group), and the patients without low C3 or low C4 (NC group).
Outcomes
At the time of biopsy, we measured the glomerular (Serum creatinine, 24h proteinuria, urinary erythrocytes) and tubular parameters (urinary n-acetyl-β-d-glucosaminidase (NAG), urinary osmotic pressure). Glomerular filtration rate was estimated using the MDRD equation for Chinese population.Citation22,Citation23 The slides were reviewed based upon the definition of the Oxford IgAN classification in the parameters including mesangial hypercellularity, endocapillary hypercellularity, segmental glomerulosclerosis, tubular atrophy/interstitial fibrosis, crescents.Citation24,Citation25 The degree of immunofluorescence was on a scale of 0 to 4 (negative, +, ++, +++, ++++). We also adopted Lee’s classificationCitation26 and the score scale by KatafuchiCitation27 and AndreoliCitation28 for the evaluation of the histopathologic injuries in general. The primary outcome was defined as the serum creatinine increased 1.5 times from baseline for the Kaplan–Meier survival analysis and Cox proportional hazard models.
Reagents
Concentrations of serum IgA, IgG, IgM, C3 and C4 were measured by a turbidimetric method using a commercial kit in commercial equipment (Beckman Coulter IMMAGE, Fullerton, CA) following the instructions from the manufacturer. Serum creatinine was measured using an enzymatic assay (SEKISUI Medical Company, Tokyo, Japan) in a biochemistry automatic analyzer (HITACHI 7180, Tokyo, Japan). 24h proteinuria was detected using a benzethonium chloride method (Cobas, Roche, Nutley, NJ) in a biochemistry automatic analyzer (HITACHI 7180, Japan). Urinary NAG was measured using a 6-methyl-2-pyridyl-N-acetyl-1-thio-d-glucosaminide method (ShenSuoYouFu Medical Diagnostic Company, Beijing, China). Urinary osmotic pressure was measured in a freezing point osmometer (Yida Medical Device Company, Zhejiang, China).
Statistical methods
Values are expressed as mean ± SD for the data with normal distribution, or median and interquartile range for the data with abnormal distribution. Differences between groups were compared using the independent t-test/general linear model for the continuous data, or the Mann–Whitney U test for the data with abnormal distribution or categorical data. A chi-square test/Fisher’s test was also adopted for the analysis of categorical data with a Mantel–Haenszel method for the test for trend. A correlation analysis was used to assess the relationship between C4 level as a continuous variable and the other parameters. A Kaplan–Meier survival analysis was performed to compare the survival rate using a log-rank test. Cox proportional hazard models were fit to explore the independent prognostic value, including age, MAP, eGFR, 24h proteinuria, C3 levels (binary variable, low or not), C4 levels (binary variable, low or not), treatment (steroid/immunosuppressor(s)) and pathological parameters based on Oxford IgAN classification including endocapillary hypercellularity, mesangial hypercellularity, interstitial fibrosis/tubular atrophy, segmental glomerulosclerosis, crescents using a backward method. Variables with p < 0.25 in log rank tests were retained as potential predictors in Cox multivariable models. All analyses were performed using SAS 9.2 (SAS, Cary, NC). A p value less than 0.05 was considered significantly different.
Results
Participants
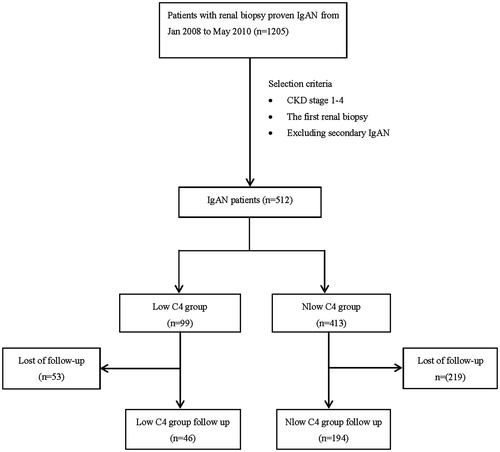
A total of 512 patients with biopsy-proven IgAN were separated into the LowC4 group (99 cases, 19.34%) and the NlowC4 group (413, 80.66%) by serum C4 levels, or separated into the LowC3 group (113, 22.07%) and the NlowC3 group (399, 77.93%) by C3 levels. These patients were also divided into LC4 group (43 patients, 8.40%), LC3 group (57 cases, 11.13%), BL group (56 cases, 10.94%), and NC group (356 cases, 69.53%) for a subgroup analysis. 240 cases (46 in the LowC4 group, 194 in the NlowC4 group) were available for following up for a median of 38.4 (interquartile range: 33.0, 44.8) months ().
Baseline clinical characteristics of the IgAN patients with low serum C4
In the LowC4 group, 24h proteinuria, urinary erythrocytes, NAG, eGFR and urinary osmotic pressure were better compared with the NlowC4 group even adjusted by age and MAP (The adjusted p value was 0.004 for eGFR, 0.001 for 24h proteinuria, 0.02 for urinary erythrocytes, <0.001 for urinary NAG and 0.02 for the urinary osmotic pressure). Both serum C4 and C3 levels were lower in the LowC4 group than those in the NlowC4 group (p < 0.05) ().
Table 1. Characteristics of IgAN patients with low level of serum C4 compared with those without low level of serum C4.
By the histopathologic evaluation according to the Oxford IgAN classification, mesangial hypercellularity, segmental glomerulosclerosis, tubular atrophy/interstitial fibrosis, crescents were all better in the LowC4 group compared with the NlowC4 group. Interestingly, the severer of these histopathologic injuries were, the lower likelihood of these injuries were in the LowC4 group as compared with the NlowC4 group (Supplementary table 1). Overall, renal pathological injuries were significantly attenuated in the LowC4 group compared with the NlowC4 group by the Lee’s classification, the Katafuchi and the Andreoli scale (). The above results suggested a renal protective role of low C4 in IgAN at the time of renal biopsy.
Renal C4 deposition was detected in 0 of 92 cases(0%) in the LowC4 group as compared with 26 of 398 cases(6.53%) in the NlowC4 group (p = 0.01), whereas IgM deposition in the LowC4 group was increased significantly (p = 0.02). No significant difference was found in the deposition of IgA, IgG, and C3 between these two groups ().
Concerning the essential role of C3 in the complement pathway and the close relationship between C4 and C3, the characteristics of IgAN patients with low C3 levels were also analyzed. Proteinuria and urinary NAG were lower in the LowC3 group than those in the NlowC3 group, and MAP and urinary erythrocytes were increased in the LowC3 group compared with the NlowC3 group. However, after adjusted by MAP that was associated with the proteinuria and renal injury, the difference of proteinuria (p = 0.1), urinary erythrocytes (p = 0.06) and urinary NAG (p = 0.1) between the two groups was insignificant. For the histopathologic evaluation, renal C3 deposition was decreased in the LowC3 group compared with the NlowC3 group. No significant difference in the other parameters was observed between these two groups (Supplementary table 2). This indicated that the low C3 levels were not associated with the renal injury in IgAN at the time of renal biopsy.
Subgroup analysis at baseline of renal biopsy
A subgroup analysis was performed to further explore the role of low C4/C3 levels on IgAN (). Twenty-four hours proteinuria, urinary NAG, eGFR and urinary osmotic pressure as well as pathological parameters including mesangial hypercellularity, segmental glomerulosclerosis, tubular atrophy/interstitial fibrosis were better in the two groups with low C4 levels (LC4 group and BL group) compared with the NC group. Overall, renal histopathologic injuries were attenuated in the LC4 group compared with the other groups by Lee’s classification, Katafuchi and Andreoli scale (active or chronic). However, these parameters were not better in the LC3 group compared with the NC group. Urinary osmotic pressure and mesangial hypercellularity were even worse in the LC3 group compared with the NC group. Andreoli chronic score was higher in the LC3 group than that in the NC group.
Table 2. Subgroup analysis of the characteristics of IgAN patients.
Correlation analysis
By a spearman correlation analysis, serum C4 as a continuous variable correlated positively to MAP, 24h proteinuria, NAG, serum IgA, serum C3, segmental glomerulosclerosis, tubular atrophy/interstitial fibrosis, Lee’s classification, Katafuchi score and Andreoli chronic score (p < 0.05), whereas correlated negatively to eGFR, serum IgM and deposition of IgG, IgM and C3 (p < 0.05) (supplementary table 3).
Follow up outcomes
The characteristics of the 240 follow-up patients at baseline of renal biopsy were shown in the . Proteinuria was lower in the follow-up subgroup than that in the loss to follow-up subgroup in the NLowC4 group (p < 0.001). All the other clinical characteristics are similar between the follow-up and the loss to follow-up subgroup in either of the LowC4 and the NlowC4 group. The clinical characteristics at baseline were attenuated in the LowC4 group compared with the NlowC4 group in the 240 follow-up patients, which was similar to the data of the whole cohort. The treatments including ACEI/ARB and immunosuppressive therapy that may affect the outcome were comparable between the LowC4 group and the NlowC4 group in the follow-up cohort.
Table 3. Clinical characteristics of the follow-up patients compared with the lost to follow-up patients.
A Kaplan–Meier survival analysis indicated that the accumulated survival rate of follow-up patients in the LowC4 group (4 cases(8.7%) developed the primary outcome of serum creatinine increased 1.5 times from baseline) was lower than that in the NlowC4 group(4 cases(2.06%) developed primary outcome) (p = 0.05, ). No significant difference of the accumulated survival curve was detected between the LowC3 group and the NlowC3 group (p = 0.4, Supplementary figure 1). By the logrank tests, the parameters with p < 0.25 including low C4 levels (p = 0.05), Proteinuria (p = 0.02), mean arterial pressure (p = 0.17), segmental glomerulosclerosis (p = 0.01), interstitial fibrosis/tubular atrophy (p = 0.09), crescents (p = 0.15), treatment by steroid (p = 0.04), treatment by immunosuppressor(s) (p = 0.01) were retained for the multivariable Cox regression analysis. eGFR, an essential confounder for the renal outcome, was also included in the model though the p value for the logrank test is 0.5. Finally, by a multivariable Cox proportional hazard model, low serum C4 (HR 9.69; 95% CI 1.90–49.48; p = 0.03), eGFR (HR 0.84; 95% CI 0.70–0.99; p = 0.04), and segmental glomerulosclerosis (HR 10.38; 95% CI 1.79–60.30; p = 0.03) were suggested to be the independent factors associated with the primary outcome ().
Figure 2. A Kaplan–Meier renal survival curve suggested the accumulative survival rate was lower in the LowC4 group than that in the NlowC4 group (p = 0.05).
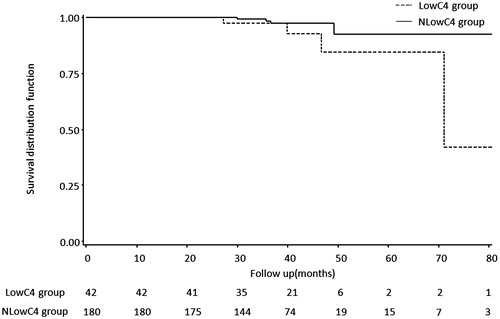
Table 4. Univariate and multivariate analysis of independent prognosis factors for the primary outcome.
Discussion
To our knowledge, the present study was the first to investigate the characteristics of the IgAN patients with low C4 levels in a large cohort for a follow-up period of more than three years. Interestingly, a high prevalence of low serum C4 was detected in the IgAN patients. These patients with low C4 levels exhibited an unusual pattern of renal presentations: They have lighter renal injury at the time of renal biopsy, whereas might be associated with a poor outcome.
Deficiency of the two isotopes of human C4 genes (C4A or C4B), associated with the low levels of serum C4,Citation29,Citation30 is the most commonly inherited immune deficiency in humans.Citation11 Approximately 35% of individuals among all races surveyed have either a C4A null or a C4B null allele; about 8–10% do not express two of the four C4 alleles, and about 1% express only a single C4 allele.Citation31 In IgAN and Henoch–Schönlein purpura nephritis patients, the frequency of homozygous null C4 phenotypes is increased.Citation17 13.9% (47/338) of Henoch–Schönlein purpura patients was reported to present with low C4 levels.Citation32 In the present study, a high prevalence (19.34%) of low C4 levels was also detected in the IgAN patients.
C4-mediated lectin pathway is involved in IgAN providing evidences as follows: in IgAN, polymeric IgA could bind to the mannan-binding lectin domain to activate lectin pathway resulting in renal C4 deposition.Citation8 The activation of lectin pathway in glomeruli indicates severer renal lesion in IgAN.Citation33 In the current study, low C4 levels were associated with better renal parameters at renal biopsy, which was validated by a correlation analysis to show that a higher C4 level correlated to a severer renal injury. This association has also been demonstrated in previous studies in animal models.Citation34,Citation35 The neutrophil recruitment in kidney and proteinuria were alleviated in the C4-deficient mice with either anti-glomerular basement membrane (GBM)-mediated nephritisCitation36 or acute nephrotoxic serum nephritis.Citation37 Though C3 is an essential factor in complement system, the analyses of the patients with low C3 levels and the subgroup analysis showed that C3 levels were not significantly associated with renal injury at baseline.
By survival analysis, the accumulated survival curve in the LowC4 group was worse than that in the NlowC4 group. Low C4 levels were independent predictive factor to increase the risk of developing primary outcome by nearly 10 folds by Cox multivariate analysis. Though treatments with ACEI/ARB and/or steroids/immunosuppressors (s) may also influence the outcome, in the present study, treatments were comparable between the patients with low C4 levels and those without, and were not a confounder by the Cox regression models. The above results indicated that low C4 levels might be associated with a poor outcome in IgAN, which was consistent with the previous reports that IgAN patients with C4A deficiency exhibit a poor prognosis.Citation38
How to explain the contradiction between the lighter renal injury at baseline and the poor outcome in the low C4 IgAN patients? C4 binds to IgM to remove the immune-complex deposition,Citation39,Citation40 which may accumulate under a C4 deficiency condition.Citation41 This may explain the increased renal IgM deposition in those with low C4 levels and the negative correlation between serum C4 and renal deposition of IgM in the current study. The gradually accumulated renal IgM deposition is associated with heavy proteinuria,Citation42 crescents formation,Citation43,Citation44 increased mesangial matrix as well as mesangial and perimesangial electron-dense depositsCitation45 leading to a poor outcome in IgAN.Citation46 We presumed that the lectin pathway may not be activated sufficiently under the condition of C4 deficiency, which protect the glomeruli from the initial injury at the time of renal biopsy. However, the gradually accumulated IgM and immune-complex deposition that cannot be removed by C4 may result in chronic glomeruli injury via promoting PDGF, TGF-β, and TLR-4 leading to a poor outcome.Citation47 In addition, C4 plays a defensive role against virus and encapsulated bacteria.Citation48,Citation49 C4 deficiency may increase the infection chances such as acute otitis media, sinusitis, or pneumoniaCitation49–51 which are the exacerbating factors for IgAN resulting in a poor prognosis.Citation52–54
Someone may presume that the low C4 levels here could be caused by the activation of complement cascade. However, C4 activation will lead to its deposition in renal tissue to aggravate glomeruli injury.Citation55 In the present study, C4 deposition was not found in anyone of the LowC4 group who presented with lighter renal injury.
There was a limitation in this study that about half of the patients were lost to follow-up. Though the characteristics of the 240 followed patients were similar to those of the whole 512 patients at baseline, the low C4 levels for the long term prognosis in IgAN may need a further long period investigation to confirm.
In summary, a high prevalence of low C4 levels was found in the IgAN patients. This cohort with low C4 levels exhibited a special pattern of renal injury. They presented with lighter renal injury at the time of renal biopsy, whereas might be associated with a poor outcome.
Supplementary tables
Download MS Word (24.8 KB)A Kaplan–Meier renal survival curve suggested there was no difference of the accumulative survival rate between the LowC3 group and the NlowC3 group (P=0.4).
Download TIFF Image (63 KB)Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by grants from the project of Hangzhou Medical Scientific Technology (2011Z013); Hangzhou Science and Technology Bureau Foundation: (no. 20080333Q17); Zhejiang Provincial Health Department financed project (no. 2008A135); the project of Zhejiang Natural Science Foundation (no.Y2101410); The project of Hangzhou Medical Scientific Technology (no. 2005Z007); The project of Zhejiang Traditional Chinese Medical Scientific Technology (no. 2005Y015). Vlado Perkovic was supported by a Heart Foundation Career Development Award. Bin Zhu was kindly supported by a fellowship from international society of nephrology (ISN). This study was also supported by Cheng Xiao-Xia studio in Hangzhou Hospital of Traditional Chinese Medicine.
References
- Simon P, Ramee MP, Boulahrouz R, et al. Epidemiologic data of primary glomerular diseases in western France. Kidney Int. 2004;66:905–908
- Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–923
- Coppo R, Amore A, Peruzzi L, Vergano L, Camilla R. Innate immunity and IgA nephropathy. J Nephrol. 2010;23:626–632
- Soto K, Wu YL, Ortiz A, Aparicio SR, Yu CY. Familial C4B deficiency and immune complex glomerulonephritis. Clin Immunol. 2010;137:166–175
- Bogers WM, Stad RK, van Es LA, Daha MR. Immunoglobulin A: Interaction with complement, phagocytic cells and endothelial cells. Complement Inflamm. 1991;8:347–358
- Wyatt RJ, Kanayama Y, Julian BA, et al. Complement activation in IgA nephropathy. Kidney Int. 1987;31:1019–1023
- Rifai A, Chen A, Imai H. Complement activation in experimental IgA nephropathy: An antigen-mediated process. Kidney Int. 1987;32:838–844
- Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol. 2001;167:2861–2868
- Endo M, Ohi H, Ohsawa I, Fujita T, Matsushita M. Glomerular deposition of mannose-binding lectin (MBL) indicates a novel mechanism of complement activation in IgA nephropathy. Nephrol Dial Transplant. 1998;13:1984–1990
- Matsuda M, Shikata K, Wada J, et al. Deposition of mannan binding protein and mannan binding protein-mediated complement activation in the glomeruli of patients with IgA nephropathy. Nephron. 1998;80:408–413
- Sjoholm AG. Inherited complement deficiency states: Implications for immunity and immunological disease. APMIS. 1990;98:861–874
- Blanchong CA, Zhou B, Rupert KL, et al. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. J Exp Med. 2000;191:2183–2196
- Truedsson L, Bengtsson AA, Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity. 2007;40:560–566
- Jin DK, Kohsaka T, Koo JW, Ha IS, Cheong HI, Choi Y. Complement 4 locus II gene deletion and DQA1*0301 gene: Genetic risk factors for IgA nephropathy and Henoch-Schönlein nephritis. Nephron. 1996;73:390–395
- Wopenka U, Thysell H, Sjoholm AG, Truedsson L. C4 phenotypes in IgA nephropathy: Disease progression associated with C4A deficiency but not with C4 isotype concentrations. Clin Nephrol. 1996;45:141–145
- Welch TR, Berry A, Beischel LS. C4 isotype deficiency in IgA nephropathy. Pediatr Nephrol. 1987;1:136–139
- McLean RH, Wyatt RJ, Julian BA. Complement phenotypes in glomerulonephritis: Increased frequency of homozygous null C4 phenotypes in IgA nephropathy and Henoch-Schönlein purpura. Kidney Int. 1984;26:855–860
- Shen PC, He LQ, Tang Y, Wang Q, Wang W, Li J. Clinicopathological characteristics and prognostic factors of asymptomatic IgA nephropathy. J Investig Med. 2010;58:560–565
- Shen P, He L, Li Y, Wang Y, Chan M. Natural history and prognostic factors of IgA nephropathy presented with isolated microscopic hematuria in Chinese patients. Nephron Clin Pract. 2007;106:c157–c161
- Cheng J, Zhang X, Zhang W, He Q, Tao X, Chen J. Efficacy and safety of glucocorticoids therapy for IgA nephropathy: A meta-analysis of randomized controlled trials. Am J Nephrol. 2009;30:315–322
- Kawasaki Y. The pathogenesis and treatment of IgA nephropathy. Fukushima J Med Sci. 2008;54:43–60
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470
- Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944
- Roberts IS, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556
- Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545
- Lee SM, Rao VM, Franklin WA, et al. IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol. 1982;13:314–322
- Katafuchi R, Kiyoshi Y, Oh Y, et al. Glomerular score as a prognosticator in IgA nephropathy: Its usefulness and limitation. Clin Nephrol. 1998;49:1–8
- Andreoli SP, Bergstein JM. Treatment of severe IgA nephropathy in children. Pediatr Nephrol. 1989;3:248–253
- Wu F, Jiang XD, Xiu-Zhu Z. The measurement of C4 concentration for different genotype in Chinese patients. J Immunol. 1992;8:256–259
- Welch TR, Beischel L, Berry A, Forristal J, West CD. The effect of null C4 alleles on complement function. Clin Immunol Immunopathol. 1985;34:316–325
- Hauptmann G, Tappeiner G, Schifferli JA. Inherited deficiency of the fourth component of human complement. Immunodefic Rev. 1988;1:3–22
- Lin Q, Min Y, Li Y, et al. Henoch-Schönlein purpura with hypocomplementemia. Pediatr Nephrol. 2012;27:801–806
- Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734
- Kouki P, Marsh JE, Sacks SH, Sheerin NS. Autoimmune renal injury in C3- and C4-deficient mice: A histological and functional study. Nephron Exp Nephrol. 2004;96:e14–e22
- Foltz CJ, Cork LC, Winkelstein JA. Absence of glomerulonephritis in guinea pigs deficient in the fourth component of complement. Vet Pathol. 1994;31:201–206
- Sheerin NS, Springall T, Carroll MC, Hartley B, Sacks SH. Protection against anti-glomerular basement membrane (GBM)-mediated nephritis in C3- and C4-deficient mice. Clin Exp Immunol. 1997;110:403–409
- Hebert MJ, Takano T, Papayianni A, et al. Acute nephrotoxic serum nephritis in complement knockout mice: Relative roles of the classical and alternate pathways in neutrophil recruitment and proteinuria. Nephrol Dial Transplant. 1998;13:2799–2803
- Wyatt RJ, Julian BA, Woodford SY, et al. C4A deficiency and poor prognosis in patients with IgA nephropathy. Clin Nephrol. 1991;36:1–5
- Circolo A. Binding of C4 by IgM. J Immunol. 1983;130:1012
- Gatenby PA, Barbosa JE, Lachmann PJ. Differences between C4A and C4B in the handling of immune complexes: The enhancement of CR1 binding is more important than the inhibition of immunoprecipitation. Clin Exp Immunol. 1990;79:158–163
- Quigg RJ, Lim A, Haas M, Alexander JJ, He C, Carroll MC. Immune complex glomerulonephritis in C4- and C3-deficient mice. Kidney Int. 1998;53:320–330
- Welch TR, McAdams J. Immunoglobulin M and C1q mesangial labeling in IgA nephropathy. Am J Kidney Dis. 1998;32:589–592
- Abuelo JG, Esparza AR, Matarese RA, Endreny RG, Carvalho JS, Allegra SR. Crescentic IgA nephropathy. Medicine (Baltimore). 1984;63:396–406
- Tang Z, Wu Y, Wang QW, et al. Idiopathic IgA nephropathy with diffuse crescent formation. Am J Nephrol. 2002;22:480–486
- Mustonen JT, Rantala IS, Helin HJ, Pasternack AI. IgA-IgM nephropathy. A subgroup of primary mesangial glomerulonephritis. Am J Clin Pathol. 1991;95:863–866
- Moriyama T, Shimizu A, Takei T, Uchida K, Honda K, Nitta K. Characteristics of immunoglobulin A nephropathy with mesangial immunoglobulin G and immunoglobulin M deposition. Nephrology (Carlton). 2010;15:747–754
- Glassock RJ. The pathogenesis of IgA nephropathy. Curr Opin Nephrol Hypertens. 2011;20:153–160
- Bishof NA, Welch TR, Beischel LS. C4B deficiency: A risk factor for bacteremia with encapsulated organisms. J Infect Dis. 1990;162:248–250
- Jaatinen T, Ruuskanen O, Truedsson L, Lokki ML. Homozygous deletion of the CYP21A-TNXA-RP2-C4B gene region conferring C4B deficiency associated with recurrent respiratory infections. Hum Immunol. 1999;60:707–714
- Kainulainen L, Peltola V, Seppänen M, et al. C4A deficiency in children and adolescents with recurrent respiratory infections. Hum Immunol. 2012;73:498–501
- Seppanen M, Suvilehto J, Lokki ML, et al. Immunoglobulins and complement factor C4 in adult rhinosinusitis. Clin Exp Immunol. 2006;145:219–227
- Yagame M, Tomino Y, Eguchi K, et al. Correlation between the pharyngeal secretion of IgA, secretory-IgA and free secretory component and the upper respiratory tract infections in patients with IgA nephropathy. Nihon Jinzo Gakkai Shi. 1987;29:1101–1105
- Tomino Y, Sakai H. Exacerbating factors in patients with IgA nephropathy. Semin Nephrol. 1987;7:315–317
- Miura M, Tomino Y, Suga T, Endoh M, Nomoto Y, Sakai H. Increase in proteinuria and/or microhematuria following upper respiratory tract infections in patients with IgA nephropathy. Tokai J Exp Clin Med. 1984;9:139–145
- Ohsawa I, Ishii M, Ohi H, Tomino Y. Pathological scenario with the mannose-binding lectin in patients with IgA nephropathy. J Biomed Biotechnol. 2012;2012:476739
Supplementary material available online Supplementary Tables 1–3 and figure