Abstract
Acute kidney injury (AKI) leads to chronic kidney disease. The mechanisms involved with recovery from AKI are poorly understood and molecular mediators responsible for healing and restoration of kidney function are understudied. We previously discovered differential expression of matrix metalloproteinase-8 (MMP-8) mRNA and protein in patients with severe sepsis associated AKI versus sepsis without AKI. Here, we demonstrate the involvement of MMP-8 in purely ischemic AKI. Mice subjected to 30 min of bilateral renal ischemia developed increased plasma creatinine and MMP-8 expression within 24 h versus sham controls. After an initial surge and subsequent return toward baseline, both kidney MMP-8 expression and activity exhibited a late increase (Days 5–7 post-ischemia reperfusion) in mice subjected to AKI. Neutrophil infiltration of the kidney was significantly higher after AKI in wild-type mice than in MMP-8 null mice, starting at 4 days. Additionally, MMP-8 null mice subjected to AKI demonstrated a persistent histopathologic and functional injury and worsened health (greater overall weight loss) versus wild-type cohorts after seven days. Taken together, our findings suggest that MMP-8 is involved with restoration of baseline kidney health after ischemic kidney injury and that a potential mechanism involves the interaction of MMP-8 and neutrophil recruitment to the site of injury.
Introduction
The incidence of acute kidney injury (AKI) in hospitalized patients is significant. By recent consensus stratification, AKI incidence is 36–67% of adults admitted to intensive care units (ICU), with 50–80% mortality,Citation1 and up to 10% of all children admitted to pediatric ICUs (PICU).Citation2,Citation3 While acute injury is associated with numerous poor outcomes in the short-term, longitudinal epidemiologic study demonstrates that AKI impacts long-term outcomes. AKI leads to high rates of chronic kidney disease, in both adults and children.Citation4–6 Unfortunately, the details of how hosts recover (or do not recover) from acute injury are not well understood.
The family of matrix metalloproteinases (MMPs) fulfills multiple roles in the kidney. MMPs, are collectively responsible for maintenance of extracellular matrix (ECM) protein scaffolds surrounding endothelia throughout the body, are involved in kidney fibrosis and chronic remodeling, diabetic nephropathy, polycystic kidney disease and glomerulonephritis.Citation7 MMPs also may regulate the kidney inflammatory response.Citation8 Experimental ischemic AKI appears to trigger both a profound inflammatory response [e.g., interleukin-6 (IL-6), IL-8 (KC) and macrophage inflammatory protein-2 (MIP-2)],Citation9,Citation10 and a response of the cytoskeletal regulatory proteins around the kidney mesangium [MMPs and tissue inhibitors of MMPs (TIMPs)]. The cytokines and chemokines that respond to ischemia are assumed to be involved in the propagation of tubular injury (acute) and conversely tubular recovery (chronic); however, exactly “how” this happens remains undefined.Citation11
We previously identified matrix metalloproteinase-8 (MMP-8) as one of 21 genes highly upregulated in children with AKI associated with sepsis (SSAKI); serum measurements of MMP-8 protein levels corroborated the microarray data (higher expression in SSAKI patients versus sepsis without AKI).Citation12 MMP-8, a collagenase, is expressed by a number of immune-regulatory cells and is responsible for balancing the homeostasis of collagens type I, II, III, VII and X along with several other cytoskeletal scaffold proteins (enactin, fibronectin and tenascin). Additionally, MMP-8 targets pro-inflammatory chemokines such as lipopolysaccharide-induced CXC chemokine (LIX)Citation13 and appears to be involved in the pathogenesis of sepsis.Citation14 Little is known, unfortunately, regarding the involvement of MMP-8 during or after ischemic AKI.
On this background, we hypothesized that MMP-8 would be involved in the pathogenesis of ischemic AKI. Using a non-lethal, ischemic AKI in wild-type and MMP-8 null mice (KO), we compared kidney and serum responses and demonstrate here that MMP-8 may be actually involved in histopathologic “recovery” from ischemic AKI.
Materials and methods
Animals and surgical protocol
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Cincinnati (IACUC No. 2E10079). Male C57Bl/6 mice or MMP-8 deficient mice (KO = MMP-8−/−) (C57Bl/6 background) (Jackson Laboratories, Bar Harbor, ME and as a gift of Dr Steven Shapiro, University of Pittsburgh) were utilized. Loss of the MMP-8 gene was confirmed by polymerase chain reaction using MMP-8 specific primers (wild-type 5′-3′ sequence: TGTCGGGTCCTGGTTTACATTCTG, 3′-5′ sequence: AAGGTCAGGGGCGATGCTACA, knock out 3′-5′ sequence: CGCCTTCTTGACGAGTTCTTCTGA). All animals were maintained under specific pathogen-free conditions, kept on a 12-h light/dark cycle, and given access to standard rodent water chow and water ad libitum.
Surgical protocol
Mice aged 4–6 weeks underwent ischemic AKI as previously described.Citation15 Mice were anesthetized using 1.5% inhaled isoflurane. Abdominal areas were shaved clean and mice were placed in a nose-cone inhalation device with continuous 1.5% isoflurane–oxygen mixture (100% oxygen at 0.8 L/min flow). Body temperature was maintained at 37 °C using a water-bath controlled heating pad underneath the surgical field and temperature was monitored intermittently using an anorectal thermometer. A horizontal midline incision was made through the skin and then the fascia. The renal pedicles were exposed using cotton-tipped blunt dissection, isolated using vessel loops and clamped bilaterally using smooth non-traumatic microvascular clamps (Roboz Surgical Instruments, Gaithersburg, MD) for 30 min. Occlusion was confirmed by visualizing renal cyanosis. A piece of gauze with warm saline was placed over the open abdominal wound during surgery. After clamp removal, reperfusion was visualized with disappearance of cyanosis to each kidney. The fascia and skin were then closed individually with continuous 4.0 Vicryl sutures. Animals were given 0.5 ml subcutaneous normal saline after the procedure and recovered on the heating pad before returning to cages. Following surgery, mice were sacrificed at indicated times per experiment for procurement of biological specimens. Mice followed to 7 days were weighed and assessed every day (and a percent weight loss or gain was calculated). Approximately 8–12 mice were used per group in each experimental condition.
Sample preparation
Blood was fractionated using centrifugation at 10,000 rpm × 10 min into plasma and whole RBC. Plasma was stored at −80 °C until use. Tissue samples were excised as indicated above and prepared for assays as listed for myeloperoxidase (MPO) and cytosol extraction.
MPO activity
MPO activity was determined as an index of neutrophil accumulation in selected tissues as previously described.Citation15 Briefly, excised kidney was homogenized MPO was assessed by spectrophotometry and defined as the quantity of enzyme degrading 1 µmol of hydrogen peroxide per min at 37 °C and expressed in units per 100-mg weight of tissue.
Subcellular fractionation and nuclear protein extraction
Kidney tissue samples were homogenized and fractionated into cytosolic and nuclear extract compartments by the manner previously described.Citation15
Serum creatinine measurements
A colorimetric-based assay (Genzyme diagnostics, Framingham, MA) was used to determine serum creatinine (SCr) concentration. Values were plotted against a standard curve after calibration and measurement at 545 nm.
MMP-8 expression and activity
MMP-8 expression was measured in both plasma and the cytosol of whole-kidney homogenate using Luminexx® xMAP multiplex array system technology (Luminexx Corporation, Austin, TX). MMP-8 activity in both plasma and kidney cytosol was determined using a commercially available fluorometric kit, according to the manufacturer’s specifications (AnaSpec Inc., Fremont, CA). The kit utilizes a FRET peptide, which is cleaved by active MMP-8, producing a measurable fluorescence at 490/520 nm.
Histologic examination of kidney tissue
A scoring system for histopathology of kidney tissue was used as described previously.Citation16 After staining with hematoxylin–eosin, kidney cross-sections were scored based on degree of: tubular cast formation, necrosis and dilation. Cross-sections were scored by four independent reviewers, all of whom were blinded to group and experimental conditions.
Weight loss (severity of illness)
Changes from baseline weight were calculated by computing difference between AKI and sham (based on percentage change from time of surgery) for each strain of mouse (WT or KO).
Statistical analysis
Results expressed in means are included with standard deviations while non-parametric results are expressed as medians with interquartile ranges. Unpaired t-tests were used as tests of significance for normally distributed data while the Mann–Whitney test was used as a test of significance for non-parametric data. To compare AKI-operated animals versus sham controls, mean values for sham operated animals were calculated and used to normalize the AKI groups (for both wild-type and null mice), creating fold-difference values for WT and KO (AKI vs. Sham). Standard error was then calculated from the normalized values for the AKI groups. p Value <0.05 was inferred as statistically significant when comparing similar groups. All statistical analyses were performed using SigmaStat 12.1 (Systat Software, Inc., San Jose, CA).
Results
Ischemic AKI is associated with increased MMP-8 expression and activity
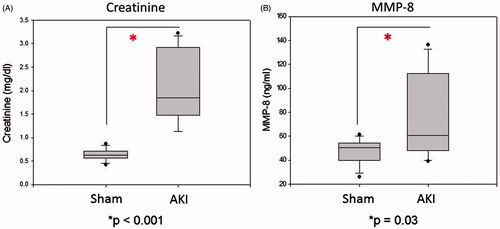
After 24 h, mice subjected to ischemia demonstrated both significantly higher SCr [1.85 (1.49, 2.91) vs. 0.62 (0.56, 0.69) mg/dL, p < 0.001) and serum MMP-8 expression [60.7 (50, 102) vs. 50.5 (40, 54) ng/mL, p = 0.03] than sham controls ().
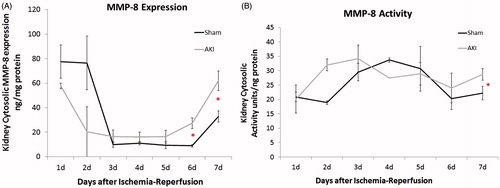
Ischemic AKI leads to a biphasic MMP-8 expression and activity response
Mice subjected to bilateral ischemia–reperfusion injury demonstrated increases in both MMP-8 expression and activity over a period of 7 days (). Mice subjected to AKI had significantly higher MMP-8 expression versus sham controls (p < 0.01) (). Whole-kidney cytosolic MMP-8 expression exhibited a steady increase in expression from Days 5–7 (peak at Day 7: 1.87 ± 0.78-fold increase when normalized to sham) (figure not shown). Similarly, MMP-8 activity remaining significantly elevated above initial post-operative levels at Day 7 and was higher in AKI mice than sham operated mice (peak at Day 7: 1.3 ± 0.09-fold increase when normalized to sham) ().
Figure 2. Kidney MMP-8 expression and activity demonstrate biphasic changes after ischemic kidney injury. Following mice 7 days after injury, wild-type mice subjected to ischemic AKI demonstrate a MMP-8 higher expression (A) and activity (B) when normalized to sham operated controls. Standard deviation after normalization is depicted. n = 6–8 for each group. (A) *p = 0.02; (B) *p = 0.04.
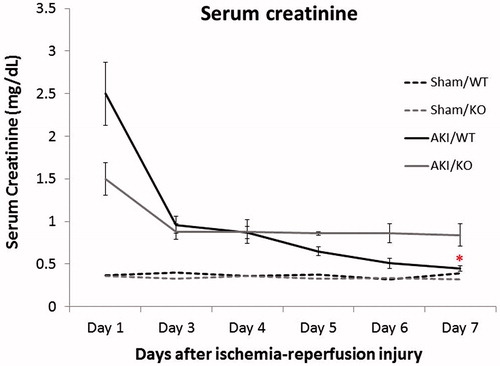
Mice lacking MMP-8 suffer persistently elevated SCr
Both WT and MMP-8 null cohorts of mice had significantly elevated creatinine in the initial post-operative period with subsequent decrease. However, SCr levels in wild-type mice returned to a near 1:1 normalization to sham operated controls, while levels in MMP-8 null mice remained elevated (2.8 ± 0.8-fold vs. 1.2 ± 0.2-fold, p < 0.01) 7 days post-injury ().
Loss of MMP-8 is associated with persistent structural injury after AKI
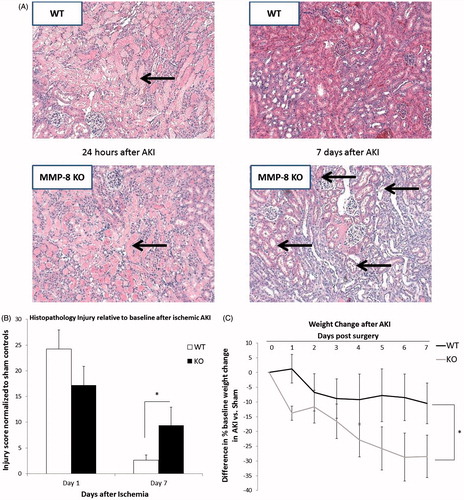
Seven days after ischemia–reperfusion injury, MMP-8 null mice displayed greater histopathologic damage than wild-type mice (). Representative cross-sections demonstrate a persistent tubular dilation and cast formation in the MMP-8 null AKI mice (compared to both sham and to both wild-type groups). Independent scoring of the cross-sections confirms a worsened injury for MMP-8 nulls relative to wild-type mice (9.4 ± 3.5-fold vs. 2.6 ± 0.9-fold, p < 0.01) (). Additionally, mice lacking MMP-8 suffered persistent weight loss seven days after AKI versus wild-type AKI mice (). The ischemic surgery did not cause mortality in any of the mice, MMP-8 null or wild-type.
Figure 4. Loss of MMP-8 is associated with persistent structural AKI and worsened overall health. (A) MMP-8 null mice demonstrate persistent tubular dilation, cast formation and peritubular necrosis (arrows) versus sham operated controls (lower right versus upper right). The wild-type mice after AKI recover to a similar extent as sham operated animals. (B) Independent review of representative cross-sections from kidneys shows that mice lacking MMP-8 had worsened histopathologic injury after 7 days (though similar after 24 h). (C) Mice lacking MMP-8 suffered persistent weight loss (and failure to regain weight) versus wild-types (values shown are absolute difference in percentage weight difference from baseline with sham controls). n = 6–8 per cohort, *p = 0.01 for 4(B) and *p = 0.04 for 4(C).
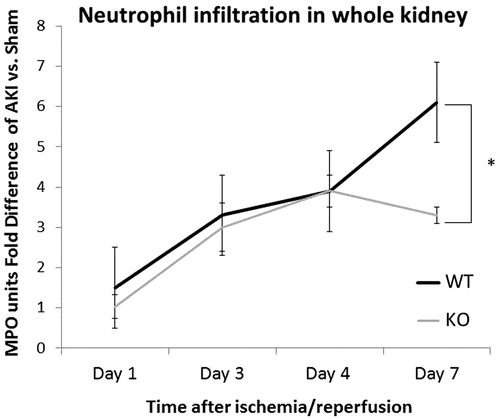
MMP-8 null mice demonstrate an altered inflammatory response after ischemia–reperfusion injury
Neutrophil infiltration, as measured by MPO activity, was significantly reduced in MMP-8 null mice versus wild-type mice at 7 days, displaying a marked divergence after 4 days. Sham controls demonstrated a nearly two-fold increase in MPO activity after AKI than nulls on Day 7 (6.1 ± 0.5-fold vs. 3.3 ± 0.2-fold, p < 0.03) ().
Discussion
Our work indicates potential involvement MMP-8 in the pathogenesis, and recovery, from ischemic AKI. Produced by neutrophils and other cell types, MMP-8 serves as a regulator of both extra-cellular matrix turnover and inflammatory signaling. After ischemia, the up-regulation of MMP-8 expression and activity in the wild-type kidney coupled with the abrogated functional and structural recovery after AKI in MMP-8 null mice suggest a critical role for MMP-8 in the recovery from ischemic injury.
Precise roles for each of the MMPs in the pathogenesis of kidney disease have not been fully detailed and available data are conflicting. The most commonly studied MMPs in kidney disease are MMP-2 and MMP-9. Both gelatinases and capable of degrading collagen type IV found in the basement membranes of glomeruli, the absence and/or inhibition of MMP-2 and MMP-9 have been associated with reduced kidney injury, improved kidney recovery and improved overall survival from AKI.Citation17,Citation18 Conversely, specific MMP-9 elimination confers a state of worsened injury on mice in models of folic acid and ischemic AKICitation19 and in models of nephritis.Citation20 Broad spectrum inhibition of MMP activity (and TNF-α converting enzyme) in a model of murine renal ischemia–reperfusion injury conferred more protection from damage than vehicle.Citation21 Unfortunately, data regarding specific MMP inhibition is limited. Select experiments with null mice have demonstrated reduced fibrosis and epithelial–mesenchymal transition (EMT) in a murine ureteral obstruction model in MMP-9 nulls,Citation22 reduced incidence of AKI after ischemia in MMP-2 nulls,Citation17 and decreased proteinuria after Adriamycin induced glomerular injury in MMP-13 nulls.Citation23 Aside from MMP-2, 9 and 13, however, there is a paucity of data regarding select MMP inhibition in kidney disease.
The specific role of MMP-8 in host responses to injury has not been fully explored. Previous work by our group has implicated MMP-8 in sepsisCitation14 and in SSAKI,Citation12 which underscores the role that MMP-8 may play in the inflammatory process. Models of aseptic inflammation (non-AKI) have indicated in a limited and inconsistent fashion that MMP-8 may help regulate acute inflammation. In a model of TNF-α mediated hepatic failure, MMP-8 null mice are protected against neutrophil infiltration and hepatocyte apoptosis.Citation24 In this study, the mechanism of protection was attributed to the reduced cleavage (LIX). Other studies indicate that MMP-8, elaborated from polymorphonuclear leukocytes (PMNs) contribute to a pro-inflammatory state.Citation25 Meanwhile, contradictory data suggests an alternate role for MMP-8. Comparisons of wild-type and MMP-8 null mice exposed to intratracheal LPS, bleomycin or hyperoxia indicates that null mice have greater lung neutrophilic infiltration, implicating MMP-8 involvement in protection from, rather than propagation of, inflammation.Citation26
In our findings of the acute inflammatory response to ischemic AKI, MMP-8 expression and activity appear to be directly proportional with recovery. Recovery observed in wild-type mice occurs in conjunction with normal or elevated MMP-8 expression and activity in the kidney. Conversely, MMP-8 null mice exhibit a blunted recovery with persistent functional and structural injury. The mechanism of MMP-8 involvement in this repair is not directly answered by our current experiments. A potential explanation for the biphasic response of MMP-8 activity and expression is the mediation of chronic repair rather than either propagation of or a reparative response to acute injury. Though we demonstrate a difference in neutrophil infiltration in the kidney in wild-type versus null animals, the chemotactic expression profile would suggest a propensity for increased inflammatory cell presence remote from the time of initial ischemic injury. Though neutrophils seem involved, it is unclear whether the neutrophils are exacerbating injury or mediating recovery. Although neutrophils are more commonly implicated in the acute pro-inflammatory process (i.e., destructive), neutrophils in uremic conditions may be integral to protection or recovery. The protective effects of uremic neutrophils have previously been demonstrated by others.Citation27,Citation28 Uremic neutrophils transferred into non-uremic mice resulted in attenuated pulmonary recruitment after an acute lung injury, thought to be secondary to altered surface expression of l-selectin.Citation28 In these experiments, the conclusion was that uremia attenuated pulmonary neutrophil recruitment. Neutrophil depletion has been shown to attenuate MMP-8 associated collagen resorption and liver repair.Citation29 The later stages of AKI more commonly feature T-lymphocytes and macrophages in the repair and salvage after inflammatory injury, but these two cell lines demonstrate significant differences between wild-type and null animals in our experiments.
MMP-8 may be involved in the transition to chronic kidney disease after ischemic AKI. MMP-8 null mice had persistently elevated creatinine versus their wild-type counterparts. The impressive persistent histopathologic injury suggest that the multiple roles of MMP-8 (collagenase, ECM protease, chemokine) lost in the null mice may be conferring a phenotype where the host is unable to mount the appropriate compensatory response and unable to properly trigger and recruit the required cellular mediators of remodeling and functional recovery. These cardinal features of aberrant ECM regulation are hallmarks of transitioning toward a pro-fibrotic and chronic kidney disease phenotype. The ratio of tissue inhibitor of metalloproteinase (TIMP) to MMP may also be intimately involved with the balance of repair or perpetual damage. The late increases in MMP-8 activity and expression supports a role in chronic remodeling and repair rather than mediation of acute events. The cellular mediation of such a response is unclear.
Our study is the first to demonstrate the biological involvement of MMP-8 in ischemic kidney injury. The only previous studies examining the role of MMP-8 in kidney disease have been limited to those involving broad based inhibition of all MMP activity (using doxycycline or tetracycline inhibition). In this series of experiments, we were able to describe a potential role for MMP-8 in the pathogenesis and recovery from ischemic AKI. If further work is able to corroborate our findings, MMP-8 may actually represent a pharmacologic target for augmenting recovery from AKI.
There are two major limitations to our study. First, as discussed earlier, the precise mechanism of MMP-8 in AKI remains unclear. Second, the source of MMP-8 in the kidney is uncertain. Our data suggests that the interplay of neutrophils and MMP-8 is important for recovery after ischemic AKI, but the mechanics of the interplay are incompletely answered. Alternatively, MMP-8 is involved in EMT, ECM maintenance, and collagen regulation, both intratubular and peritubular. The production of MMP-8 by tubular epithelial cells in the kidney and from other non-neutrophil sources remains unexplored. Together, the absence of MMP-8 may alter many different potential mechanisms, some of which are not addressed here, required for the proper homeostatic balance regulating repair and recovery.
Selective MMP inhibition offers promise to identify a specific role for MMP-8, but a more in-depth exploration of MMP-8 substrates and breakdown products in AKI will likely yield great insight into the explicit behaviors of all MMPs in AKI.Citation30,Citation31 Net proteolytic activity triggered by MMPs is a complex series of interactions between not only each MMP and its targeted substrate, but also between MMPs, tissue inhibitors of MMPs (TIMPs), and other protease/protease inhibitor families.Citation32,Citation33 Further study of the role of MMPs and TIMPs in AKI will determine their aggregate role in maintaining the balance between injury and repair. The identification and study of putative mediators of repair after AKI is equally important to the identification and study of mediators of injury after AKI.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported, in part, by grants from the National Institutes of Health: (P50 DK096418 and R01GM096994).
References
- Hoste EA, Schurgers M. Epidemiology of acute kidney injury: How big is the problem? Crit Care Med. 2008;36(Suppl. 4):S146–S151
- Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38(3):933–939
- Basu RK, Devarajan P, Wong H, Wheeler DS. An update and review of acute kidney injury in pediatrics. Pediatr Crit Care Med. 2011;12(3):339–347
- Devarajan P. Pediatric acute kidney injury: Different from acute renal failure but how and why. Curr Pediatr Rep. 2013;1(1):34–40
- Goldstein SL, Jaber BL, Faubel S, Chawla LS, Acute Kidney Injury Advisory Group of American Society of Nephrology. AKI transition of care: A potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol. 2013;8(3):476–483
- Kline J, Rachoin JS. Acute kidney injury and chronic kidney disease: It’s a two-way street. Ren Fail. 2013;35(4):452–455
- Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Ren Physiol. 2012;302(11):F1351–F1361
- Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19(1):34–41
- Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74(7):901–909
- Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19(3):547–558
- El Sabbahy M, Vaidya VS. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip Rev Syst Biol Med. 2011;3(5):606–618
- Basu RK, Standage SW, Cvijanovich NZ, et al. Identification of candidate serum biomarkers for severe septic shock-associated kidney injury via microarray. Crit Care. 2011;15(6):R273
- Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem. 2003;270(18):3739–3749
- Solan PD, Dunsmore KE, Denenberg AG, Odoms K, Zingarelli B, Wong HR. A novel role for matrix metalloproteinase-8 in sepsis. Crit Care Med. 2012;40(2):379–387
- Basu RK, Donaworth E, Wheeler DS, Devarajan P, Wong HR. Antecedent acute kidney injury worsens subsequent endotoxin-induced lung inflammation in a two-hit mouse model. Am J Physiol Renal Physiol. 2011;301(3):F597–F604
- Mishra J, Mori K, Ma Q, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15(12):3073–3082
- Kunugi S, Shimizu A, Kuwahara N, et al. Inhibition of matrix metalloproteinases reduces ischemia-reperfusion acute kidney injury. Lab Invest. 2011;91(2):170–180
- Lee SY, Horbelt M, Mang HE, et al. MMP-9 gene deletion mitigates microvascular loss in a model of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2011;301(1):F101–F109
- Bengatta S, Arnould C, Letavernier E, et al. MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol. 2009;20(4):787–797
- Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco PM. Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med. 2001;193(7):793–802
- Novak KB, Le HD, Christison-Lagay ER, et al. Effects of metalloproteinase inhibition in a murine model of renal ischemia-reperfusion injury. Pediatr Res. 2010;67(3):257–262
- Wang X, Zhou Y, Tan R, et al. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2010;299(5):F973–F982
- Sakamaki Y, Sasamura H, Hayashi K, et al. Absence of gelatinase (MMP-9) or collagenase (MMP-13) attenuates Adriamycin-induced albuminuria and glomerulosclerosis. Nephron Exp Nephrol. 2010;115(2):e22–e32
- Van Lint P, Wielockx B, Puimege L, Noel A, Lopez-Otin C, Libert C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J Immunol. 2005;175(11):7642–7649
- Rajasekhar L, Liou LB, Chan CY, Tsai WP, Cheng CY. Matrix metalloproteinase-8 in sera and from polymorphonuclear leucocytes in rheumatoid arthritis: In vitro characterization and correlation with disease activity. Clin Exp Rheumatol. 2004;22(5):597–602
- Quintero PA, Knolle MD, Cala LF, Zhuang Y, Owen CA. Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 alpha to reduce acute lung inflammation and injury in mice. J Immunol. 2010;184(3):1575–1588
- Singbartl K. Renal-pulmonary crosstalk. Contrib Nephrol. 2011;174:65–70
- Zarbock A, Schmolke M, Spieker T, Jurk K, Van Aken H, Singbartl K. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: A critical role for uremic neutrophils. J Am Soc Nephrol. 2006;17(11):3124–3131
- Harty MW, Muratore CS, Papa EF, et al. Neutrophil depletion blocks early collagen degradation in repairing cholestatic rat livers. Am J Pathol. 2010;176(3):1271–1281
- Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803(1):39–54
- Overall CM, Dean RA. Degradomics: Systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2006;25(1):69–75
- Overall CM. Molecular determinants of metalloproteinase substrate specificity: Matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol. 2002;22(1):51–86
- Overall CM. Matrix metalloproteinase substrate binding domains, modules and exosites. Overview and experimental strategies. Methods Mol Biol. 2001;151:79–120