Abstract
Ischemic acute renal failure is a condition that extends subsequent to sudden and momentary fall in overall or regional blood flow to the kidney. The present investigation was deliberated to scrutinize the renoprotective potential of berberine in animal model of renal ischemia reperfusion (RIR) induced dent via assessment of various biochemical and molecular biomarkers. Male Wistar rats were anesthetized and the right kidney was removed through a small flank incision. Renal ischemia reperfusion was persuaded in uni-nephrectomized rats by occlusion of left renal artery for 45 min and reperfusion for 4 weeks. After 4 weeks of treatment of berberine (10, 20, and 40 mg/kg, p.o.), hemodynamic and left ventricular function were evaluated. Induction of ischemia reperfusion resulted callous mutilation in kidney which was confirmed by alterations in oxidative stress (SOD, GSH, and MDA), membrane bound enzymes, kidney function markers (serum creatinine and BUN), and mitochondrial dysfunction. Moreover, RIR injury exhibited incredible alterations in mRNA expression of KIM-1, NGAL, Caspase-3, Bax, Bcl-2, and TNF-α levels. Conversely treatment of berberine (20 and 40 mg/kg) significantly (p < 0.01 and p < 0.001) restored ischemia reperfusion induced marring via intonation of biochemical and molecular biomarkers. To sum up, berberine demonstrated compelling renoprotective effect in RIR injury via caspase-mitochondria-dependent pathway.
Introduction
Renal ischemia is a principal source of acute renal failure (ARF) and results in high rates of morbidity and fatality. Furthermore, acute renal injury is a common health problem with increasing outrageous incidences and still meagre salutary preferences.Citation1 Renal ischemia reperfusion is a multifaceted disorder concerning diverse mechanisms characteristically renal vasoconstriction, insidious tubular injury, and glomerular damage.Citation2 Moreover, ischemia reperfusion injury entails assorted proceedings, including hammering of energy, deformation of the ionic hemostasis, generation of impulsive oxygen species, and cell demise. Due to acute renal injury, there is a swift demur in renal function pigeonholed by accretion of nitrogenous wastes such as blood urea nitrogen and creatinine. It has been ascribed that anoxia, discharge of abrupt oxygen species like superoxide radicals, hydrogen peroxide, and hydroxyl radicals and neutrophils accrual are the elementary corollaries of ischemia reperfusion injury.Citation3 It is revealed that during renal transplantation, aortic, and open heart surgery, renal ischemia reperfusion contributes a substantial dilemma.Citation4
The pathophysiology of acute renal failure demonstrates the multifaceted connection between hemodynamics, tubular injury, and inflammation.Citation5 In addition, dent to vascular endothelial cells result dysregulation of various systems like vascular tone, vasopermeability, and coagulation which caused interstitial congestion and curbed blood flow and leukocyte permeation.Citation6 It has been accredited that the impulsive momentary drop in total or provincial blood flow to the kidney urges to prompt an ischemic renal failure.Citation7 Even though ensues in precautionary approaches and support measures renovascular hypertension continues to be allied with extensive adversities.Citation8,Citation9 In addition, it is mentioned that acute renal failure is a consequence of renal ischemia, which endorsed copious and fused sequence of measures, ensuing an injury, and escorted the renal cells to decease.Citation10 Despite the fact that reperfusion is essential for survival of ischemic tissue, it is acknowledged that remarkable quantity of reactive oxygen species and adenosine 59-triphosphate are spawned by reperfusion itself. Furthermore, rationale of calcium is also paramount in pathophysiology of renal damage instigated by hypoxia and ischemia reperfusion.Citation11
Berberine is an isoquinoline alkaloid and present in ample plants in the Berberis and Coptis genera which demonstrates broad range of pharmacological assets.Citation12 It has been acknowledged that berberine has a strapping antitumor and antioxidant potential.Citation13 It has been ascribed that berberine has potential to assuage gastrointestinal infections, inflammatory responses, carcinoma, and diabetes.Citation14 Berberine illustrated influential consequences on cardiovascular diseases by reinstating cardiotoxicity, recuperating cardiac dysfunction, arrhythmia, and atherosclerosis.Citation15,Citation16 Numerous pollsters have reported salutary prospective of berberine as compassionate in the management of hypertension, atherosclerosis, and heart disease, together with left ventricular remodeling.Citation17–19 It is demonstrated that berberine concealed expression of transforming growth factor-β (TGF-β) in renal tissue to alleviate renal dysfunction and decelerate the development of diabetic nephropathy in rats.Citation20 Berberine exerts its renoprotective effect via modulating G proteins-AC-cAMP signaling pathway in diabetic rats.Citation21 It is also reported that berberine protected hypoxia/reoxygenation injury in human renal proximal tubular cells through the obstructing endoplasmic reticulum and mitochondrial stress pathways.Citation22
After renal ischemia injury apoptosis is a chief imperative mechanism of cell fatality in cultivated renal tubular cells and exposed kidneys. Apoptosis is pigeonholed by various facets like cell shrinkage, plasma membrane snitching, chromatin abridgment, and genomic DNA crumble, which is an elementary for expansion and upholding of tissue homeostasis.Citation23,Citation24 Conversely, various vascular ailments such as congestive heart failure and ischemic injury are predominantly consequences of apoptosis. Even though it is acknowledged that necrosis implicated the cell loss during cardiac ischemia and reperfusion, there were many reports who inveterate apoptosis as a commencement of myocyte death.Citation25 The membrane structure and function is transformed by ROS, which can persuade cellular injury during lipid peroxidation reactions.Citation3 As a result, cellular degradation is hustled by tubular permeability, reduction in mitochondrial phosphorylation, and release of lysosomal enzymes, which eventually lessened renal function.Citation3 Despite of the comprehensive alien about cellular and molecular mechanisms of cell injury, it has been considered that necrosis and apoptosis of renal epithelial cells are the upshot of acute renal failure.Citation26 It has been endorsed that newly identified kidney marker kidney injury molecule-1 (KIM-1) which is customary in urine for an extended phase, nonetheless, can be distinguished in the kidney and urine in numerous animal models of nephrotoxins.Citation27 During ischemia reperfusion, mitochondria accuses permeability evolution, prompted by assorted circumstances, such as amplified outset of reactive oxygen species (ROS), diminution of antioxidants, modification of pyridine nucleotide fractions, fluctuation of Ca2+ concentration, and engorge of inorganic phosphate in the prevailing surroundings.Citation28,Citation29 Moreover, Bcl-2 family proteins stupendously diminish the apoptosis implicated through intracellular caspase-3 proteins. It is generally accorded that the mitochondria dynamically concerned in cell death through increased permeability of the membranes.Citation30 Renal ischemia reperfusion instigates inflammatory reactions like endothelial activation and damage, enhanced endothelial cell-leukocyte conformity, leukocyte snare, and conciliation in microvascular blood flow.Citation31
Even though there has been encroachment in perceptive of multifaceted measures like pathophysiological, biochemical, and molecular prospects of renal ischemia reperfusion injury in animal models, rendition of these conclusions to restorative benefits in clinical practice remains exigent. Moreover, there are few data available to support the use of berberine in RIR. Hence, the aim of present investigation was to evaluate the potential of berberine against renal ischemia reperfusion induced vascular mutilation in rat by assessing various hemodynamic, biochemical, and molecular parameters.
Materials and methods
Experimental animals and research protocol approval
Adult male Wistar rats (180–200 g) were purchased from the National Institute of Biosciences, Pune (India). They were maintained at 24 ± 1 °C, with relative humidity of 45–55% and12:12 h dark/light cycle. The animals had free access to standard pellet chow (Pranav Agro industries Ltd., Sangli, India) and water throughout the experimental protocol. All experiments were carried out between 09:00 and 17:00 h. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Poona College of Pharmacy, Pune and performed in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals (CPCSEA).
Drugs and chemicals
Berberine was purchased from Sigma Chemical Co. (St Louis, MO). 1,1′,3,3′-tetraethoxypropane, crystalline beef liver catalase, reduced glutathione (GSH), 5,5′-dithiobis (2-nitrobenzoic acid) were purchased from S.D. Fine Chemicals, Mumbai, India. Total RNA Extraction kit and One-step RT-PCR kit was purchase from MP Biomedicals India Private Limited, India.
Experimental design
Animals were divided randomly into five groups of six animals each as follows:
Group I: Sham: Non-nephrectomized rats underwent the exposure of the left renal artery, but did not receive ischemia reperfusion. They were received distilled water (10 mg/kg, p.o.) for 28 days.
Group II: RIR control: Uni-nephrectomized rats underwent left renal artery occlusion for 45 min. They were received distilled water (10 mg/kg, p.o.) for 28 days.
Group III: RIR + B (10): Uni-nephrectomized rats underwent left renal artery occlusion for 45 min. They were received berberine (10 mg/kg, p.o.) for 28 days.
Group IV: RIR + B (20): Uni-nephrectomized rats underwent left renal artery occlusion for 45 min. They were received berberine (20 mg/kg, p.o.) for 28 days.
Group V: RIR + B (40): Uninephrectomized rats underwent left renal artery occlusion for 45 min. They were received berberine (40 mg/kg, p.o.) for 28 days.
The real artery occlusion was carried out in the uni-nephrectomized rat. Briefly, rats were anesthetized on day 0, with thiopental sodium (35 mg/kg, i.p.). The abdominal region was shaved with a safety razor and sterilized with povidone iodine solution and the right kidney was removed through a small flank incision. After recovery period, small incision was given on the left side of peritoneal cavity of the uni-nephrectomized animal to expose left kidney. The left renal artery was occluded for 45 min using renal bulldog clamp and then reperfusion for 4 weeks. During this period, the animals received treatment with either distilled water or berberine (10, 20, and 40 mg/kg, p.o.) for 4 weeks. On the last day of experiment hemodynamic changes, left ventricular function were assessed, blood and renal tissue were collected for assessment of biochemical and molecular biomarkers.
Invasive measurement of hemodynamic changes
Hemodynamic changes such as heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MABP), left ventricular end diastolic pressure (LVEDP), and ventricular contractility assessment (dp/dt) were measured by means of a polyethylene cannula (PE 50) filled with heparinized saline (100 IU/mL) inserted into the right carotid artery. The cannula was connected to a transducer and the signal was amplified by means of bioamplifier. Left ventricular systolic pressure was measured by means of a Millar micro-tip transducer catheter (Model SRP-320, Millar instrument, INC 320-7051, Houston, TX) inserted into the left ventricle via the right carotid artery and connected to a bioamplifier. The left ventricular functions like dp/dtmax, dp/dtmin and left ventricular end diastolic pressure signals were obtained from primary signals (left ventricular systolic pressure and blood pressure) by means of an acquisition data system (AD Instruments Pvt. Ltd. with software LabChart 7.3; AD Instrument Pvt. Ltd, Gurgaon, Haryana).Citation32
Renal function
Blood was collected from the animals by retro-orbital puncture (ROP) and centrifuged at 7500 rpm for 15 min at 4 °C. Then serum was transferred using micropipette in Eppendorf tubes and stored at 4 °C till analyzed. Serum samples were assayed for blood urea nitrogen (BUN) and creatinine using standard diagnostics kits. Ischemia reperfused rats from second experiment were housed in metabolic cages for 24 h on last day and at the end of urine collection urine flow (µl/min/kg) was estimated.
Endogenous antioxidant enzymes in kidney
Immediately after hemodynamic measurements animal were sacrificed and left kidney were isolated and weighed. Left kidney sample from two animals were arbitrarily selected for histopathology. The kidney sample from remaining animals were cut in to small pieces, placed in chilled 0.25 M sucrose solution and blotted on a filter paper. The tissues were then homogenized in 10% chilled Tris-hydrochloride buffer (10 mM, pH 7.4) by tissue homogenizer (Remi Motors, Mumbai, India) and centrifuged at 7500 rpm for 15 min at 0 °C using Eppendorf 5810-R high speed cooling centrifuge. The clear supernatant was used for the assays of various antioxidant enzymes. The sediment was resuspended in ice cold Tris buffer (10 mM, pH 7.4) to get a final concentration of 10% and was used for the estimation of Ca2+ATPase and Na+K+ATPase. Supernatant of homogenates was employed to estimate superoxide dismutase (SOD), reduced glutathione (GSH), lipid peroxidation (MDA content) as described previously.Citation33,Citation34 Membrane bound enzymes, namely Na+K+ATPase, and Ca2+ATPase were assayed according to the previously performed methods.Citation32,Citation33,Citation35
Myeloperoxidase activity
Myeloperoxidase (MPO) activity was measured in tissues by a procedure similar to that previously documented method.Citation36
Isolation of mitochondria from kidney
Mitochondria were isolated from kidneys according to previously reported method.Citation37 Cytochrome oxidase assay was performed according to previously reported method.Citation38
Determination of KIM-1, NGAL, TNF-α, caspase-3, Bax, and Bcl-2 by reverse transcriptase PCR in kidney
RNA isolation
The levels of mRNA were analyzed in renal tissue using a reverse transcription (RT)-PCR approach as described previously.Citation39,Citation40
cDNA preparation
Single-stranded cDNA was synthesized from 5 µg of total cellular RNA using reverse transcriptase (MP Biomedicals India Private Limited, India) as described previously.Citation39 The primer sequence for KIM-1, neutrophil gelatinase-associated lipocalin (NGAL), tumor necrosis factor-α (TNF-α), caspase-3, Bcl-2-associated X protein (bax), Bcl-2, and β-actin is presented in . Amplification of β-actin served as a control for sample loading and integrity. PCR products were detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. Size of amplicons was confirmed using a 100-bp ladder as a standard size marker. The amplicons were visualized and images were captured using a gel documentation system (Alpha Innotech Inc., San Leandro, CA). Expression of all the genes was assessed by generating densitometry data for band intensities in different sets of experiments and was generated by analyzing the gel images on the Image J program (Version 1.33, Wayne Rasband, NIH, Bethesda, MD) semi-quantitatively. The band intensities were compared with constitutively expressed β-actin. The intensity of mRNAs was standardized against that of the β-actin mRNA from each sample, and the results were expressed as PCR-product/β-actin mRNA ratio.
Table 1. Primer sequences for KIM-1, NGAL, TNF-α, caspase-3, Bax, Bcl-2 and β-actin.
Histological evaluation of kidney
At the end of experiment left kidney was isolated and fixed for histopathological evaluation with 4% buffered paraformaldehyde solution and embedded in paraffin. Three to four micrometer thick paraffin sections were dewaxed and brought to water through graded ethanol. Sections were stained with hematoxylin–eosin stain (H & E), then dehydrated through graded ethanol, cleared in xylene and mounted with Distyrene Plasticizer Xylene. H & E stained sections were graded for the presence of medullary congestion, tubular cell necrosis, cytoplasmic vacuolization, glomerular hypertrophy, and cytoplasmic eosinophilia.
Statistical analysis
Data was expressed as mean ± standard error mean (SEM). Data analysis was performed using Graph Pad Prism 5.0 software (Graph Pad, San Diego, CA). Data were analyzed by one-way analysis of variance (ANOVA) and Dunnett’s tests were applied for post hoc analysis. A value of p < 0.05 was considered to be statistically significant.
Results
Effect of berberine on RIR induced alteration in kidney weight and kidney function
There was significant (p < 0.001) increased in kidney weight of RIR rats as compared to sham rats. However, treatment of berberine (40 mg/kg) showed substantial (p < 0.01) diminution in kidney weight as compared to RIR rats. Moreover, kidney function markers such as creatinine and BUN were noticeably (p < 0.001) elevated in RIR group as compared to the sham group. Conversely treatment of berberine (20 and 40 mg/kg) showed significant and dose-dependant demure in serum creatinine (p < 0.05 and p < 0.01) and BUN (p < 0.05 and p < 0.001) level as compared to the RIR group ().
Table 2. Effect of berberine on RIR induced alteration in kidney function.
Effect of berberine on RIR induced alteration in hemodynamic changes
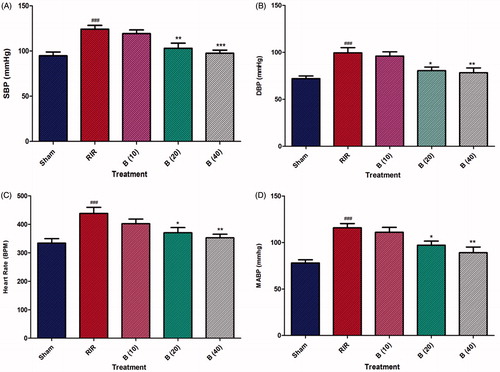
There was dramatic elevation (p < 0.001) in heart rate, SBP, DBP, and MABP of RIR rats as compared to sham rats. On the other hand, treatment of berberine (20 and 40 mg/kg) exhibited significant and dose-dependent (p < 0.05 and p < 0.01) attenuation of heart rate, SBP, DBP, and MABP as compared to the RIR group ().
Figure 1. Effect of berberine on RIR induced alteration in hemodynamic changes. Data are expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by Dunnett’s test multiple range test. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared with the renal ischemia reperfusion group. ###p < 0.001 as compared to sham control group. RIR: Renal ischemia reperfusion group; B (10): berberine (10 mg/kg, p.o.) treated group; B (20): berberine (20 mg/kg, p.o.) treated group; B (40): berberine (40 mg/kg, p.o.) treated group.
Effect of berberine on RIR induced alteration in left ventricular function
There was staggering mutilation (p < 0.001) in left ventricular function which is corroborated by the drastic sore in EDP, dp/dtmax, dp/dtmin, pressure time index and Tau of RIR rats as compared to sham rats. Administration of berberine (20 mg/kg) noticeably (p < 0.05, 0.01, 0.01, 0.05, and 0.01) decreased EDP, dp/dtmax, dp/dtmin, pressure time index, and Tau as compared to RIR group, respectively. Furthermore, treatment of berberine (40 mg/kg) significantly (p < 0.001, 0.001, 0.01, 0.001, 0.01, and 0.01) reduced the EDP, dp/dtmax, dp/dtmin, pressure time index, and Tau, respectively. Furthermore, there was significantly (p < 0.001) reduction in contractility index in RIR group which was considerably (p < 0.01) restored by the treatment of berberine (40 mg/kg; ).
Table 3. Effect of berberine on RIR induced alteration in left ventricular function.
Effect of berberine on RIR-induced alteration in oxidative stress
There was considerable (p < 0.001) demure in renal SOD and GSH level of RIR rats as compared the sham rats. However, there was significant and dose dependant elevation in renal SOD (p < 0.01 and 0.001) and GSH (p < 0.05 and 0.01) levels were observed by administration of berberine (20 and 40 mg/kg). In addition, renal MDA and MPO levels were drastically (p < 0.001) elevated in RIR group as compared to sham group. On the other hand, treatment of berberine (20 and 40 mg/kg) illustrated dose dependent and momentous (p < 0.01 and 0.001) dwindling in renal MDA levels. The elevated level of MPO was significantly and dose dependently inhibited by the berberine (10, 20, and 40 mg/kg) treatment as compared to RIR control rats ().
Table 4. Effect of berberine on RIR induced alteration in oxido-nitrosative stress, membrane bound enzymes, and cytochrome oxidase.
Effect of berberine on RIR induced alteration in membrane bound enzymes
There was significant (p < 0.001) decreased in Na-K-ATPase and increased in Ca-ATPase levels after RIR injury in RIR control rats as compared to sham rats. However, treatment of berberine (20 and 40 mg/kg) significantly restored the Na-K-ATPase (p < 0.05 and 0.01) and Ca-ATPase (p < 0.01 and 0.001) levels as compared to RIR control rats ().
Effect of berberine on RIR-induced alteration in cytochrome oxidase level
Cytochrome oxidase level was significantly increased in RIR control rats as compared to sham control rats. Treatment with berberine (20 and 40 mg/kg) significantly and dose dependently (p < 0.01 and 0.001) inhibited RIR induced increased cytochrome oxidase level as compared to RIR control rats ().
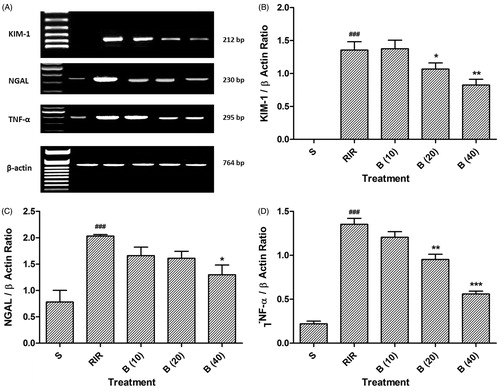
Effect of berberine on RIR-induced alteration in NGAL, KIM-1, and TNF-α mRNA expression
There was significant (p < 0.001) up-regulation in KIM-1 and NGAL mRNA expression in RIR rats as compared to sham rats. However, administration of berberine (20 and 40 mg/kg) significantly and dose dependently (p < 0.05 and 0.01) down-regulated KIM-1 mRNA expression as compared to sham group. Whereas, treatment with berberine (40 mg/kg) stupendously (p < 0.05) down-regulated renal KIM-1 expression as compared to RIR rats. There was up-regulation (p < 0.001) TNF-α mRNA expression of RIR group as compared to sham group. Administration of berberine (20 and 40 mg/kg) significantly and dose dependently (p < 0.01 and 0.001) down-regulated renal TNF-α mRNA expression as compared to RIR rats ().
Figure 2. Effect of berberine on RIR induced alteration in NGAL, KIM-1 and TNF-α mRNA expression (A), quantitative representation of mRNA expression of NGAL (B), KIM-1 (C), and TNF-α (D). Data are expressed as mean ± SEM (n = 4) and analyzed by one-way ANOVA followed by Dunnett’s test multiple range test. *p < 0.05, **p < 0.01 and ***p < 0.001 as compared with the renal ischemia reperfusion group. ###p < 0.001 as compared to sham control group. RIR: Renal ischemia reperfusion group; S: Sham control group; B (10): berberine (10 mg/kg, p.o.) treated group; B (20): berberine (20 mg/kg, p.o.) treated group; B (40): berberine (40 mg/kg, p.o.) treated group.
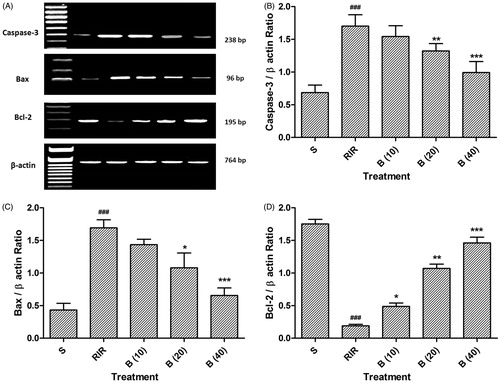
Effect of berberine on RIR-induced alteration in Bax, Bcl-2, and caspase-3 mRNA expression
There was significant (p < 0.001) up-regulation in Bax and caspase-3 expressions of RIR rats as compared to sham rats. However, administration of berberine (20 and 40 mg/kg) considerably down-regulated Bax and caspase-3 mRNA expressions as compared to RIR control rats. In addition, renal ischemia reperfusion injury reminiscently (p < 0.001) down-regulated Bcl-2 mRNA expression in RIR control rats as compared to sham rats. Conversely treatment of berberine scrupulously and dose dependently (p < 0.05, 0.01, and 0.001) escalated the mRNA expression of Bcl-2 ().
Figure 3. Effect of berberine on RIR induced alteration in caspase-3, Bax, and Bcl-2 mRNA expression (A), quantitative representation of mRNA expression of caspase-3 (B), Bax (C), and Bcl-2 (D). Data are expressed as mean ± SEM (n = 4) and analyzed by one-way ANOVA followed by Dunnett’s test multiple range test. *p < 0.05, **p < 0.01 and ***p < 0.001 as compared with the renal ischemia reperfusion group. ###p < 0.001 as compared to sham control group. RIR: Renal ischemia reperfusion group; S: Sham control group; B (10): berberine (10 mg/kg, p.o.) treated group; B (20): berberine (20 mg/kg, p.o.) treated group; B (40): berberine (40 mg/kg, p.o.) treated group.
Effect of berberine on RIR-induced alteration in kidney histology
depicted the normal architecture of kidney from sham control rats which was devoid of congestion, necrosis, and inflammatory infiltration with intact glomerulus basement membrane (grade 0). Renal ischemia reperfusion injury resulted in disruption of glomerular structural reflected by the presence of intraluminal cell debris, edema, and inflammatory infiltration (). Berberine (20 and 40 mg/kg) treated rats showed moderate number of inflammatory cells with decreased necrosis and edema. Thickens of glomerulus basement membrane was intact ( and ).
Figure 4. Effect of berberine on RIR induced alteration in kidney histology. Photomicrograph of sections of kidney of (A) normal, (B) RIR control rats, (C) Berberine (20 mg/kg) treated rats, and (D) Berberine (40 mg/kg) treated rats. H & E staining at 100×.
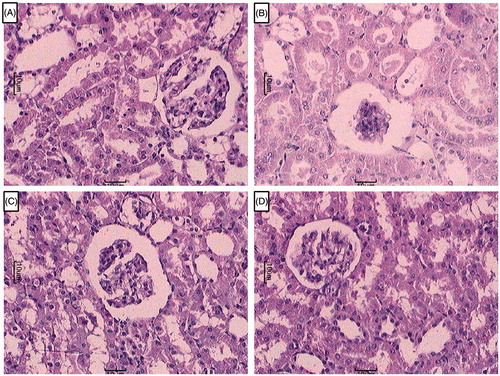
Table 5. Effect of berberine on RIR induced alteration in kidney histology.
Discussion
The various facets such as glomerulonephritis, diabetic nephropathy, and hypertensive nephrosclerosis are the diverse outburst among kidney diseases.Citation41,Citation42 Nonetheless subsequent to persuaded degree of renal mutilation, evolution of renal disease is unswerving, irrevocable, and principally independent of the preliminary slur. RIR have been implicated in the pathogenesis of renal injury induced by ischemia reperfusion by directly affecting kidney cells. Renal injury induced by ischemia reperfusion is due to an assortment of facets like hypoperfusion, hypoxia, inflammatory reactions, and free radical-induced dent.Citation43 Depending on the rigorousness of ischemia, renal artery occlusion can lead to damage and dysfunction of the kidney. Ischemic renal injury is pigeonholed by intra-renal vasoconstriction, leading to abridged glomerular plasma flow and filtration rate, and reduced oxygen deliverance to the tubules of the outer medulla.Citation44 The selected animal model of ischemia reperfusion produced hypertension by diminution in renal perfusion which is prefers extensively for a better perceptive of the rapport among the renin–angiotensin system, hypertension, and cardiovascular disorders.Citation45 This study assesses isolated bioactive moiety berberine to protect against renovascular dysfunction in laboratory animals.
Renal ischemia reperfusion leads to increased kidney weight which may be reflected by edema and apparent increases in collagen accumulation and hypertrophic growth. The results of present investigations are in line with findings of previous investigator.Citation46 A swift change in serum creatinine is the largely frequent sign of acute kidney injury. Due to excess of creatinine acute inflammatory edema and tubular necrosis formation are accompanied by significant changes in the incidence of cellular proliferation. There are ample of reports concerning decrease in glomerular filtration rate of ischemia reperfusion rats because of remarkable elevate in serum creatinine and blood urea nitrogen levels which are in accordance the earlier findings.Citation47 However, treatment of berberine appreciably ameliorated the renal function by lessening in creatinine and blood urea nitrogen.
Consequences from experimentation of renal ischemia reperfusion verified that ischemia reperfusion for 45 min lead to alterations in the rat kidney, resulting from the termination of blood supply. Equivalent degree of the renal ischemia has been fashioned by other pollsters.Citation48 After fourth week of RIR in nephrectomized rats exhibited noteworthy escalate in SBP, DBP, MABP, EDP, dp/dtmax, and dp/dtmin which are in synchronization with findings of previous researcher,Citation49 who has reported hypertension as foremost concern for expansion of acute renal diseases. In the present delve, berberine treatment illustrated considerable diminish in SBP, DBP, MABP, EDP, dp/dtmax, and dp/dtmin. Antihypertensive activity of berberine has been studied by researcher,Citation50 who has reported the antihypertensive effect of berberine is probably through a central sympatholytic effect.
Kidney is an organ which is extremely sensitive to changes in oxygen tensions within its complex architecture making it very prone to hypoxic injury. It is well known that complex architecture of kidney make it prone to hypoxic injury when renal artery is momentarily occluded, tremendous transforms in oxygen tension was measured. Furthermore, oxidative stress alters oxygen metabolism and oxygen availability.Citation51,Citation52 Ischemic injury rockets the expression of adhesion proteins which facilitate the conscription and permeation of circulating leukocytes ensuing in undulating, and adherence of leukocytes in the renal tissue.Citation53 Vascular clogging and capillary plugging are cause by neutrophils endothelial adhesion in the external strip of outer medulla. In addition, cell death is impelled by released leukocytes who unfetter auxiliary ROS, proteolytic enzymes, and cytokines.Citation54 Renal ischemia reperfusion leads to increased MPO activity due to neutrophils dissemination into kidney may be prominent than sham group. In our case, drug might lessened adhesion molecule emergence and MPO commotion, perhaps due to its antioxidative properties leading to dwindling in inflammation.
In present investigation noteworthy diminution in the activities of Na+K+ATPase and Ca2+ATPase in the renal homogenate were perceived after fourth week of renal ischemia reperfusion injury. These alterations may be ascribed to the lipid peroxidation by free radicals, while these membrane bound enzymes contain “SH” group, which are lipid reliant and their movement is prone to be flawed with the change in the composition of membrane lipids.Citation55 The berberine (40 mg/kg)-treated group illustrated noteworthy encouragement in the Na+K+ ATPase and Ca2+ATPase which may be due to the aptitude of berberine to shield the “SH” group from oxidative dent through the inhibition of peroxidation of membrane lipids.
The mRNA expression of NGAL is notably prominent in the kidney subsequent to ischemic slurCitation56 and has been flaunted to be an exceedingly prognostic biomarker of acute and chronic kidney hurts.Citation57 Recently NGAL has been described as an early, extremely perceptive and distinct renal biomarker which is fretful in the delineation of kidney epithelia. It has been evidence that simultaneous administration of NGAL with ischemia reperfusion illustrates nephroprotective effect.Citation58 Moreover, secretion of NGAL is enormously increased by kidney epithelia when it is dent by ischemia reperfusion, nephrotoxins, and sepsis which is demonstrated initially in rats, mice, pigs, and then in human neonates children and adults.Citation59,Citation60 Due to lofty materialization of NGAL being apparent in neutrophils and epithelium, NGAL stimulation is hypothesized to be the upshot of connections among inflammatory cells and the epithelial lining.Citation61
KIM-1 is a trans-membrane protein which is not noticeable in normal kidney tissue, but is uttered at incredibly elevated level in upset proximal tubule epithelial cells in human and rodent kidneys after ischemic or toxic injury.Citation62 The proximal tubule is predominantly susceptible to ischemic impairment in animal models. It has been demonstrated that KIM-1 mRNA expression was immensely amplified in ischemia reperfusion injury gradually after 10, 20, 30, or 45 min bilateral ischemia reperfusion injury. It has been exposed that KIM-1 is harmonized with the markers of epithelial cell dedifferentiation and promulgation in animals.Citation62 KIM-1 was strikingly up-regulated in post-ischemic rat kidney which was conspicuously abbreviated by berberine.
Renal ischemia reperfusion injury propels to cell death in cultured renal tubular cells and isolated kidneys which are contemptuously coupled with apoptosis. Cellular necrosis is a result of large component of cell death during myocardial ischemia reperfusion injury. In addition, latest information advocated that reperfusion stage is regarded as overall myocytes death period which is predominantly contributed by apoptosis.Citation63 Furthermore, apoptosis is plainly linked with the over expression of proapoptotic protein (Bax), downregulation of antiapoptotic protein (Bcl-2), and amplified caspase-3 activity.Citation64
Recently, it has been revealed that caspase are the brand of intracellular cysteine proteases, which contribute via two diverse signaling pathways like commencement of pro-inflammatory cytokines and endorsement of apoptotic cell demise.Citation64 Moreover, Caspase instigation is advanced dreadfully by oxidation of extracellular matrix proteins which hustle mesangial cell apoptosis and front to hypocellularity in glomerulosclerosis.Citation65 Furthermore, caspase-3 is a key fragment implicated in apoptosis and inflammation progress.Citation66 Oxidative stress is malevolently produced by renal ischemia reperfusion which have a propensity to activate caspase-3 and provokes apoptosis and necrosis to spawn detrimental desolations like renovascular dysfunction and hypertension.Citation67 It is attributed that caspase distinguished in apoptotic cells and are extensively consider intervening the functioning of apoptosis by lacerating and encumbering intracellular proteins that are requisite for cell endurance and promulgation.Citation68
It has been acknowledged that expression of TNF-α drastically augmented due to ischemic insults.Citation69 Development of renal ischemia reperfusion induced organ damage is a consequence of neutrophils activation through TNF-α. Stimulated neutrophils were concerned as contributed in the pathogenesis of I/R-induced renal injury by destructing endothelial cells and impel the release of inflammatory mediators which eventually decline the renal blood flow. Intonation of caspase-associated pro-apoptotic progression can assuage the apoptosis of cardiomyocytes owed to ischemic injury as well it may consent ischemic myocardium to endure even after receipt considerable damage. Treatment of berberine may be considerably refurbish the caspase-3 level and thwart cardiomyocytes apoptosis via hampering ROS–TNF-α–caspase signaling pathway.Citation70
Bcl-2 associated proteins managed different pro-apoptotic signal transduction and damage pathways congregate on mitochondria to provoke mitochondrial membrane penetration. Bcl-2 is acknowledged as potent inhibitor of apoptosis caused by ischemia reperfusion and concerned in prolonged cell endurance by encumbering apoptotic pathway. In addition, it is revealed that the expression of Bcl-2 family proteins and mitochondrial dysfunction are key components of the apoptotic process.Citation71 Ischemia reperfusion robustly diminished Bcl-2 and ascended TNF-α level which is correlated with caspase-3 pathway in present investigation whereas treatment of berberine prudently block caspase-3 pathway via revolutionizing reperfusion injury by up regulating Bcl-2 and hindering TNF-α level.
The deleterious effects of ischemia reperfusion on mitochondria are mainly augmented generation of ROS, diminution of antioxidants, and modification of pyridine nucleotide ratios, vacillation of Ca2+ concentration and expansion of inorganic phosphate in the matrix.Citation72,Citation73 It has been recognized that generation of oxidative stress subsequent to ischemia reperfusion injury was noticeably allied with mitochondrial DNA damage through exhaustion of thiols such as glutathione in isolated cells.Citation33,Citation74 Moreover, it is renowned that apoptosis was triggered by involvement of proteases, nucleases, and phospholipases through rise in intra-mitochondrial pHCitation75 or from calcium released from mitochondria into the cytosol.Citation76 It has been ascribed that apoptosis pathway is well-characterized by receptor arbitrated and mitochondrial-dependent pathways which ultimately stimulate the downstream slayer caspase-3. Procaspases-3 cleaved and stimulated by commencement of cell death receptor TNF-α which initiated the receptor-dependent pathway to activate procaspase-8. While cytochrome C discharged from mitochondria educe the mitochondrial dependent pathway. Many pollsters have reported an influential role of Bcl-2 genes in commencement of caspase via affect on cytochrome C release from mitochondria.Citation77 Tremendous variations within mitochondria occur as a corollary of extended ischemia, which remarkably diminished the activity of cytochrome oxidase (complex IV)Citation78 which are in accordance with our investigation.
Conclusion
It is concluded that berberine exhibited potential renoprotective effect in renal ischemia reperfusion rats. Treatment of berberine for 4 weeks after ischemia reperfusion appreciably dropped blood pressure, dp/dtmax, dp/dtmin, and pressure time index. Furthermore, berberine diminished kidney weight, creatinine, and BUN levels along with restored MPO activity and TNF-α expression in nephrectomized rat. Berberine attenuated the RAAS induced oxidative damage revealed by improved the levels of SOD, GSH, and decreased MDA level. The anti-apoptotic role of berberine was palpable from the hampering ROS–TNF-α–Caspase signaling pathway along with anti inflammatory escalating by restoring NGAL-1 and KIM-1 expression. These results designated that the renoprotective activity of berberine may be interceded through a caspase–mitochondria-dependent pathway.
Acknowledgements
The authors acknowledge Dr. S. S. Kadam, Vice chancellor, Bharati Vidyapeeth University and Dr. K. R. Mahadik, Principal, Poona College of Pharmacy for keen interest and providing the necessary facilities to carry out the study.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370
- Weinberg JM. The cell biology of ischemic renal injury. Kidney Int. 1991;39:476–500
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164
- Myers SI, Wang L, Liu F, Bartula LL. Oxygen-radical regulation of renal blood flow following suprarenal aortic clamping. J Vasc Surg. 2006;43:577–586
- Gill N, Nally JV, Fatica RA. Renal failure secondary to acute tubular necrosis epidemiology, diagnosis, and management. CHEST J. 2005;128:2847–2863
- Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460
- Gosavi T, Kandhare A, Raygude K, Ghosh P, Bodhankar S. A comparative study on the efficacy, safety and cost effectiveness of Viscum album and Rauwolfia serpentina mother tincture in hypertensive patients. Deccan J Nat Prod. 2011;2:29–35
- Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol Renal Physiol. 1996;271:F477–F488
- Paller MS. The cell biology of reperfusion injury in the kidney. J Invest Med. 1994;42:632–639
- Birdsall TC, Kelly GS. Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Altern Med Rev. 1997;2:94–103
- Pereira CV, Machado NG, Oliveira PJ. Mechanisms of berberine (natural yellow 18)–induced mitochondrial dysfunction: Interaction with the adenine nucleotide translocator. Toxicol Sci. 2008;105:408–417
- Vuddanda PR, Chakraborty S, Singh S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Exp Opin Invest Drugs. 2010;19:1297–1307
- Dong S-F, Hong Y, Liu M, et al. Berberine attenuates cardiac dysfunction in hyperglycemic and hypercholesterolemic rats. Eur J Pharmacol. 2011;660:368–374
- Hong Y, Hui SS, Chan BT, Hou J. Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci. 2003;72:2499–2507
- Xu M-G, Wang J-M, Chen L, Wang Y, Yang Z, Tao J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology. 2008;112:279–286
- Zeng X-H, Zeng X-J, Li Y-Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92:173–176
- Lee S, Lim H-J, Park H-Y, Lee K-S, Park J-H, Jang Y. Berberine inhibits rat vascular smooth muscle cell proliferation and migration in vitro and improves neointima formation after balloon injury in vivo: Berberine improves neointima formation in a rat model. Atherosclerosis. 2006;186:29–37
- Tang L, Lv F, Liu S, Zhang S. Effect of berberine on expression of transforming growth factor-beta1 and type IV collagen proteins in mesangial cells of diabetic rats with nephropathy. Zhongguo Zhong Yao Za Zhi. 2011;36:3494–3497
- Tang LQ, Wang FL, Zhu LN, Lv F, Liu S, Zhang ST. Berberine ameliorates renal injury by regulating G proteins-AC- cAMP signaling in diabetic rats with nephropathy. Mol Biol Rep. 2013;40:3913–3923
- Yu W, Sheng M, Xu R, Yu J, Cui K, Tong J, et al. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J Transl Med. 2013;11:24–34
- Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact. 2014;219C:101–112
- Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Elucidation of molecular mechanism involved in neuroprotective effect of Coenzyme Q10 in alcohol induced neuropathic pain. Fundam Clin Pharmacol. 2013;27:603–622
- Kajstura J, Cheng W, Reiss K, et al. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107
- Wei Q, Wang MH, Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. Am J Nephrol. 2005;25:491–499
- Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–F563
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83:650–659
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett. 2012;511:18–22
- Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/Life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642
- Willinger CC, Schramek H, Pfaller K, Pfaller W. Tissue distribution of neutrophils in postischemic acute renal failure. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:237–243
- Visnagri A, Kandhare AD, Ghosh P, Bodhankar SL. Endothelin receptor blocker bosentan inhibits hypertensive cardiac fibrosis in pressure overload-induced cardiac hypertrophy in rats. Cardiovasc Endocrinol. 2013;2:85–97
- Kandhare AD, Raygude KS, Shiva Kumar V, et al. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomed Aging Pathol. 2012;2:173–186
- Kandhare AD, Bodhankar SL, Singh V, Mohan V, Thakurdesai PA. Anti-asthmatic effects of type-A procyanidine polyphenols from cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed Aging Pathol. 2013;3:23–30
- Kandhare A, Raygude K, Ghosh P, Bodhankar S. The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Int J Green Pharm. 2011;5:236–243
- Kandhare AD, Ghosh P, Ghule AE, Zambare GN, Bodhankar SL. Protective effect of Phyllanthus amarus by modulation of endogenous biomarkers and DNA damage in acetic acid induced ulcerative colitis: Role of phyllanthin and hypophyllanthin. Apollo Med. 2013;10:87–97
- Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab. 1987;7:752–758
- Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria a biochemical and morphological study. J Cell Biol. 1967;32:415–438
- Kandhare AD, Shivakumar V, Rajmane A, Ghosh P, Bodhankar SL. Evaluation of the neuroprotective effect of chrysin via modulation of endogenous biomarkers in a rat model of spinal cord injury. J Nat Med. 2014;68(3):586–603
- Visnagri A, Kandhare AD, Chakravarty S, Ghosh P, Bodhankar SL. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol. 2014;52:814–828
- Ghosh P, Kandhare AD, Raygude KS, et al. Determination of the long term diabetes related complications and cardiovascular events using UKPDS risk engine and UKPDS outcomes model in a representative western Indian population. Asian Pac J Trop Dis. 2012;2:S642–S650
- Kamble H, Kandhare AD, Bodhankar S, Mohan V, Thakurdesai P. Effect of low molecular weight galactomannans from fenugreek seeds on animal models of diabetes mellitus. Biomed Aging Pathol. 2013;3:145–151
- Mejia-Vilet JM, Ramirez V, Cruz C, Uribe N, Gamba G, Bobadilla NA. Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am J Physiol Renal Physiol. 2007;293:F78–F86
- Cotran R, Rennke M, Kumar V. The kidney and its collecting system. In: Robbins SL, Kumar V, eds. Basic Pathology. Philadelphia: WB Saunders Company; 1987:457–491
- Pickering TG, Mann S. Renovascular hypertension: Medical evaluation and nonsurgical treatment. In: Laragh JH, Brenner BM, eds. Hypertension: Pathophysiology, Diagnosis, and Management. New York: Raven Press; 1990:2–14
- Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: Long-term histology of cell and matrix changes in the rat. Kidney Int. 2000;57:2375–2385
- Korkmaz A, Kolankaya D. Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J Surg Res. 2010;164:309–315
- Hagiwara S, Koga H, Iwasaka H, et al. ETS-GS, a new antioxidant, ameliorates renal ischemia-reperfusion injury in a rodent model. J Surg Res. 2011;171:226–233
- Aunapuu M, Pechter U, Kuhnel W, Ots M, Arend A. Morphological changes in experimental postischemic rat kidney. A pilot study. Ann Anat. 2005;187:63–70
- Liu JC, Chan P, Chen YJ, Tomlinson B, Hong SH, Cheng JT. The antihypertensive effect of the berberine derivative 6-protoberberine in spontaneously hypertensive rats. Pharmacology. 1999;59:283–289
- Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28:132–145
- Raygude KS, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology. 2012;20:331–341
- Mizuno S, Nakamura T. Prevention of neutrophil extravasation by hepatocyte growth factor leads to attenuations of tubular apoptosis and renal dysfunction in mouse ischemic kidneys. Am J Pathol. 2005;166:1895–1905
- Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107
- Lieberthal W, Koh JS, Levine JS. Necrosis and apoptosis in acute renal failure. Semin Nephrol. 1998;18:505–518
- Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543
- Gaspari F, Cravedi P, Mandalà M, et al. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: A pilot prospective case-control study. Nephron Clin Pract. 2010;115:c154–c160
- Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621
- Huynh TK, Bateman DA, Parravicini E, et al. Reference values of urinary neutrophil gelatinase-associated lipocalin in very low birth weight infants. Pediatr Res. 2009;66:528–532
- Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery – A prospective cohort study. Crit Care Med. 2009;37:553–560
- Carlson M, Raab Y, Seveus L, Xu S, Hällgren R, Venge P. Human neutrophil lipocalin is a unique marker of neutrophil inflammation in ulcerative colitis and proctitis. Gut. 2002;50:501–506
- Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244
- Olivetti G, Quaini F, Sala R, et al. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–2016
- Waller HL, Harper SJ, Hosgood SA, et al. Differential expression of cytoprotective and apoptotic genes in an ischemia‐reperfusion isolated organ perfusion model of the transplanted kidney. Transpl Int. 2007;20:625–631
- Mattana J, Kochlatyi S, Gibbons N. Metal-catalyzed oxidation of extracellular matrix proteins promotes human mesangial cell apoptosis and is associated with enhanced expression of Bax and caspase activation. Biochem Biophys Res Commun. 2002;292:652–658
- Yang B, Jain S, Ashra SY, Furness PN, Nicholson ML. Apoptosis and caspase-3 in long-term renal ischemia/reperfusion injury in rats and divergent effects of immunosuppressants. Transplantation. 2006;81:1442–1450
- Kunduzova OR, Escourrou G, Seguelas M-H, et al. Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J. 2003;17:872–874
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424
- Daemen MA, van de Ven MW, Heineman E, Buurman WA. Involvement of endogenous interleukin-10 and tumor necrosis factor-alpha in renal ischemia-reperfusion injury. Transplantation. 1999;67:792–800
- Lv X-X, Yu X-H, Wang H-D, et al. Berberine inhibits norepinephrine-induced apoptosis in neonatal rat cardiomyocytes via inhibiting ROS-TNF-α-caspase signaling pathway. Chin J Integr Med. 2013;19:424–431
- Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620
- Adil M, Visnagri A, Kumar VS, Kandhare AD, Ghosh P. Protective effect of naringin on sodium arsenite induced testicular toxicity via modulation of biochernical perturbations in experimental rats. Pharmacologia. 2014;5:222–234
- Kandhare AD, Raygude KS, Ghosh P, et al. Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pac J Trop Biomed. 2012;5:337–344
- Goswami S, Kandhare A, Zanwar AA, et al. Oral l-glutamine administration attenuated cutaneous wound healing in Wistar rats. Int Wound J. 2014 [advance online publication]. doi: 10.1111/iwj.12246
- Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273:C1783–C1792
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911
- Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol. 1983;244:H743–H748

