Abstract
Background: The aim of the present study is to investigate the impact of de novo donor-specific antibodies (dnDSA) on early graft function, to provide objective reference for early clinical diagnosis and reasonable individualized treatment. Methods: 305 cases of renal transplant patients for the first time were observed in this study. Follow-up time for all recipients was 6 months after operation. HLA antibody, DSA, renal function were monitored after transplant. Results: In total of 305 cases, 66 cases (21.64%) were HLA antibody positive and 21 cases (6.89%) showed acute rejection (AR) in 6 months after transplant. The HLA antibody-positive patients included six cases of dnDSA-positive and 60 cases of dnDSA-negative. The incidence of AR was 2.09% (5/239) in HLA antibody-negative patients, 18.33% (11/60) in HLA antibody positive with DSA-negative patients, and 83.33% (5/6) in HLA antibody-positive patients with DSA-positive. There was a big difference between DSA-negative and DSA-positive patients (p < 0.01). The recovery time of AR patients with DSA-positive were longer than DSA-negative patients, and the recovery graft function of AR patient with DSA-positive were not as good as those with DSA-negative. Conclusions: The appearance of dnDSA in the early stage of kidney transplantation is a warning sign of AR occurrence. Dynamic monitoring of HLA antibody and DSA could predict the state of graft function, and play an important role in the prevention of AR, timely and effectively.
Introduction
Kidney transplantation is currently the best treatment for patients with end-stage renal disease.Citation1 In the most recent era, the global transplantation community has progressively taken into account the importance of antibody-mediated rejection (ABMR) as evidenced by the amount of publications on the subject (more than 3500 articles referenced in Pub Med to date). We all know the antibodies which induced ABMR are derived from the graft stimulation of the host immune system. So, the less HLA matching is the major way to reduce the HLA antibody production after transplantation.
HLA matching is one of the highlights of long-term research in the field of organ transplant. It plays an important role on recipients choosing, effectively preventing and reducing rejection occurrence and improving the transplant survival rate in the organ transplantation. With the development of organ transplant technology, there has been a further understanding of the function and meaning on HLA matching. The more HLA mismatching number, the greater chance of panel reactive antibody (PRA) positive of transplantation group.
PRA estimates the likelihood of positive cross-matches to potential donors.Citation2 The technology of PRA assay has advanced from the initial complement-dependent cytotoxicity (CDC) assay, to the enzyme-linked immunoabsorption (ELISA), to the current multiplexed particle-based flow cytometry (Luminex; One Lambda Inc., Canoga Park, CA). Single-antigen beads are increasingly used to characterize the preformed HLA antibodies before transplant as well as any de novo development of HLA antibodies (donor-specific antibodies, DSA) after transplant.Citation3 Measurement of DSA has been recently proposed as another important tool to monitor the humoral counterpart of the alloimmune status. The development of DSA post-transplantation has been associated with higher graft failure rates and is thought to represent a sign of poor immunosuppression.Citation4–6
At present, more data show the vital relations between DSA and the survival time of graft. Lachmann et al. summarized the 5-year survival rate of the 1014 cases patients after kidney transplantation in Europe, and found the presence of DSA was associated with a significantly lower graft survival of 49% versus 83% in the HLAab-negative group. Non-DSAs also had an adverse effect on graft survival (70% vs. 83%).Citation7 A report in 2012 also studied 315 transplants without pretransplant DSA in North America. The median 10-year graft survival for those with de novo DSA (dnDSA) was lower than the dnDSA group (57% vs. 96%, p < 0.0001). Pathology consistent with antibody-mediated injury can occur and progress in patients with dnDSA in the absence of graft dysfunction, and furthermore, non-adherence and cellular rejection contribute to dnDSA development and progression to graft loss.Citation6
Currently, there are no data regarding the relationship of performed dnDSAs and graft function of recipients in 6 months after transplant. In consideration of the role of DSAs in graft dysfunction, the aim of the present study is to document the incidence of dnDSAs in our patient and investigate the impact of dnDSAs on early graft function recovery including the assessment of the level of dnDSAs, the graft function recovery, and the occurrence of acute rejection in early post-transplant.
Materials and methods
Patients’ population
From May 2012 to May 2014, 308 patients received kidney transplantation at the First Affiliated Hospital of Xi’an Jiaotong University (Xi’an, China). From this original group, three recipients were excluded because of two transplantations or lost follow-up, leaving 305 patients to be included in this study. Follow-up time for all recipients was 6 months after operation. AR was confirmed by either biopsy-proven rejection or clinical rejection of the kidney with a favorable response to anti-rejection treatment. This study has been cleared by our Institution Ethics Review Board for human studies and that patients have signed an informed consents.
HLA typing and epitope mismatch identification
High-resolution HLA typing (HLA-A, -B, -C, -DR and -DQ) was performed using sequence-specific oligonucleotide probes (LABType_HD SSO, One Lambda, Canoga Park, CA). HLA-A, -B, and -DR (three pairs, six antigens) are used for matching before kidney transplant. All patients were complement-dependent cytotoxicity cross-match (CDC-XM) negative and therefore considered as low risk for early acute rejection (AR).
Patient management
During the transplant operation and 1–5 d post-operation, the shock treatment was adopted by applying anti-human thymocyte globulin (ATG) (75 mg/d) and methyl-prednisone (Pred; 250 mg/d). All of the recipients were treated with the Cyclosporin A (CsA)/Tacrolimus (FK506) + MMF + prednisone regimen. The initial dose of CsA was 4.5–5.0 mg/kg/d. FK506 was 0.1 mg/kg/d. Mycophenolate mofetil (MMF) was taken orally on the first day after the transplant at a dosage of 1.5–2.0 g/d. CsA/FK506 and Pred were administered synchronously, with the initial dose of Pred 40 mg/d and the maintenance dose of 15–20 mg/d.
For treatment AR, the regimen was switched to use ATG or plasma exchange treatment when Pred shock therapy failed. CsA/FK506 + MMF + prednisone regimen was reused after AR reversed.
HLA antibody test
Pre- and post-transplant sera were screened for anti-HLA antibodies using single purified HLA antigen-coated synthetic flow beads (LABScreen™ single-antigen beads, One Lambda, Canoga Park, CA) according to the manufacturer’s protocol. To identify the DSA specificities, the donor–recipient mismatched HLAs were compared to the antibody profile for each patient’s sample. DSAmax is defined as the antibody specificity that arises first during post-transplantation. If multiple antibody specificities arise at the same sample point, the antibody with the highest MFI is considered the DSAmax. The time points for DSA dynamic testing were 3 d, 5 d, 1 week, 2 weeks, 3 weeks,1 month, 2 months, 3 months, and 6 months after transplant. The median fluorescence index (MFI) value of more than 500 was decided for positive results.
C1q test
Serum samples from patients with circulating donor-specific anti-HLA antibodies were analyzed in a blinded fashion at the Beijing Mentality biological technology company for the presence of C1q-binding donor-specific anti-HLA antibodies with the use of single-antigen flow bead assays according to the manufacturer’s protocol (C1qScreenTM, One Lambda).Citation8
Diagnosis of acute rejection
All reported rejections were biopsy-proven and done only when a patient’s serum creatinine increased by at least 20% over baseline. The biopsy specimens were evaluated by light microscopy and immunofluorescence for C4d according to the Banff 2007 classification.Citation9
Data analysis
Data were processed using the SPSS17.0 software package for Windows (SPSS Inc., Chicago, IL). Chi-square test or Fisher exact test was used to compare categorical data. Mann–Whitney U test and Student t-tests were used as appropriate for continuous data. Results were considered statistically significant if p < 0.05.
Results
General data
This retrospective survey study was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University and was performed in compliance with the Declaration of Helsinki. A total of 305 cases of kidney transplantation were involved in this study. The general data of donors and recipients were showed in . The average age of the recipients was 36 ± 7.5 years. The primary diseases were including chronic glomerulonephritis (58.0%), hypertensive nephropathy (13.8%), membranous nephropathy (12.1%), IgA nephropathy (10.2%), diabetes (3.3%), and other diseases with unknown etiology (2.6%). There were 295 cases receiving hemodialysis and 10 cases receiving peritoneal dialysis before the transplant operation.
Table 1. Demographics of recipients and donors.
HLA-A, B, and DQ matching results showed that most cases were two or three antigen mismatches, except seven cases of five antigen mismatches, 21 cases of four antigen mismatches, 30 cases of one antigen mismatches, and four cases of zero antigen mismatches (). For all cases, the ABO blood types between donors and recipients were compatible and the cross-matches were all negative.
Table 2. HLA matches of donor and recipient.
Condition of HLA antibody before and after kidney transplantation
The data of HLA antibodies monitoring show that 17 patients with HLA antibody-positive (11 cases of HLA I and 6 cases of HLA II antibody) and 288 cases with HLA antibody-negative pre-operative (). After transplantation, it was found that 66 cases were HLA antibody-positive (51 cases of HLA I, 28 cases of HLA II, and 13 case of HLA I + HLA II antibody) including six cases dnDSA-positive (). Among dnDSA-positive patients (six cases), four cases were from HLA antibody positive cases pre-operative, the positive rate is 23.53%. However, only two cases were from HLA antibody-negative cases pre-operative, the positive rate is 0.69% (), indicating the pre-operative HLA antibody-positive was a major risk factor for inducing dnDSA after transplant.
Table 3. Condition of HLA antibody before and after kidney transplantation.
Table 4. Relationship of pre-operative HLA antibodies and DSA after kidney transplantation.
DSA/NDSA monitoring of kidney transplant recipients
To investigate the level of DSA and non-donor specific antibodies (NDSA), 305 cases of recipients were monitored dynamically after transplantation. Recipients of both DSA and NDSA antibodies positive were in all six cases, accounting for 1.97%; recipients with DSA antibody negative and NDSA antibody positive were 60 cases, accounting for 19.67%; recipients with both DSA and NDSA antibody negative were 239 cases, accounting for 78.36% (). As shown in , DSA or NDSA were detected about in 2 weeks after transplantation in 66 cases of HLA-positive recipients, in which DSAmax or NDSAmax MFI of 54 cases were fluctuated between 500 and 5000, and then in a relatively stable state; DSAmax or NDSAmax MFI of increased to 5000–10,000 in seven cases and increased to more than 10,000 in five cases, then fell to below 5000 after anti-rejection therapy or oral immunosuppressant drugs.
Figure 1. DSA/NDSA monitoring of kidney transplant recipients. DSA or NDSA were detected in 6 months after transplantation in 66 cases of HLA-positive recipients, 54 cases of recipients DSAmax or NDSAmax MFI fluctuated between 500 and 5000, and then in a relatively stable state; seven cases of recipients DSAmax or NDSAmax increased to 5000–10,000; 5 cases of recipients DSAmax or NDSAmax increased to more than 10,000, then fell to below 5000 after anti-rejection therapy or oral immunosuppressant drugs.
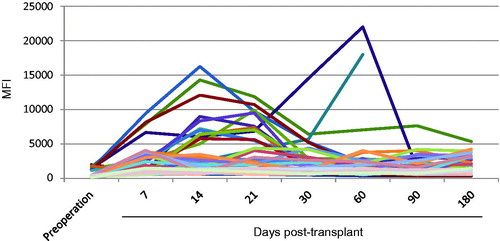
Table 5. DSA/NDSA monitoring of kidney transplant recipients.
Relationship of HLA antibody with AR occurrence
The dynamic monitoring of HLA antibody showed that 239 patients were HLA antibody-negative (DSA−/NDSA−), 60 cases were DSA−/NDSA+ patients, and 6 cases were DSA-positive patients (DSA+/NDSA+). The AR occurrence of each group was 2.09%, 18.33% and 83.33%, respectively. There was significant difference (p < 0.01) between DSA − /NDSA− recipients and DSA−/NDSA + and DSA+/NDSA+ recipients. Moreover, the difference was also found between DSA−/NDSA+ and DSA+/NDSA+ (p < 0.01) ().
Table 6. Relationship of HLA antibody with AR occurrences.
Relationship of HLA antibody with recovery time and graft function of AR
In all 305 cases, 21 cases were AR, and the incidence of AR was 6.89%. Most rejection occurred in 5–20 d after transplantation. Nineteen cases were successfully reversed after treatment with ATG induced for 5–8 d or when given plasma exchange. The unrecovered three cases were included; two cases of grafts excision and one case of acute heart failure caused death.
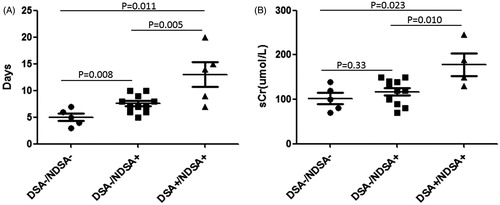
The recovery time of AR patients was 5 ± 1.6 d for patients with DSA−/NDSA−, 7.5 ± 2.0 for patients with DSA−/NDSA+, and 13 ± 5.1 for patients with DSA+/NDSA+. There were significant differences between each group (). The graft function after anti-rejection treatment was 101.8 ± 28.3 μmol/L, 117.2 ± 28.2 μmol/L, and 178.5 ± 50.7 μmol/L for DSA−/NDSA−, DSA−/NDSA+, and DSA+/NDSA+ patients, respectively (). The differences were only found between group DSA+/NDSA+ and other two groups. Taken together, the recovery time of AR patients with DSA-positive were longer than DSA-negative patients, and the recovery graft function of AR patient with DSA-positive were not as good as those with DSA-negative.
Figure 2. Relationship of DSA with recovery time and graft function of AR. (A) The recovery time of AR patients with DSA−/NDSA−, DSA−/NDSA+, or DSA+/NDSA+. There were significant differences between each groups. (B) The graft function after anti-rejection treatment for groups DSA−/NDSA−, DSA−/NDSA+, and DSA+/NDSA+. The differences were only found between group DSA+/NDSA+ and other two groups.
Clinical data of DSA-positive patients
All the DSA-positive recipients were included as: men in one case and women in five cases. The HLA mismatch of case 1 was 5, for other cases, the mismatch was 3 or 2. The primary disease of most cases was chronic glomerulonephritis (Case 1, 2, 4, 5). For case 3 and case 6, the primary diseases were hypertensive nephropathy and IgA nephropathy. Only cases 4 and 6 were HLA antibody-negative before transplant. The dnDSA of case 1, 4, and 5 were HLA class I antibody. Case 2 was HLA class II antibody. Cases 3 and 6 were HLA class I and II antibodies. C4d staining of all cases was positive except case 5. We also found that C1q of case 1, 2, and 6 were positive and for other cases were negative (). As show in , only case 4 did not show AR and recovered smoothly. We also found that the DSA MFI value of case 4 was waved around 3000, but the peak DSA MFI value of other cases were higher than 10,000. We think the value of DSA MFI is the very important reason for inducing AR. Four of the AR cases were reversed after treatment with ATG or when given plasma exchange (Case 1,2,5,6). Case 3 was dead because of complication such as lung infection, uncompleted cardiopulmonary function.
Figure 3. Relationship of DSA MFI and AR occurrence and reverse. (A–F) Case 1 to 6 with DSA-positive. Black line: the Creatinine dynamic changes of recipients post-operative. Blue line: the DSA MFI dynamic changes of recipients post-operative. Green line: The time of treatment. The below form described the HLA matching of patient (PT) and donor (DN). Red words: HLA locus which induced DSA.
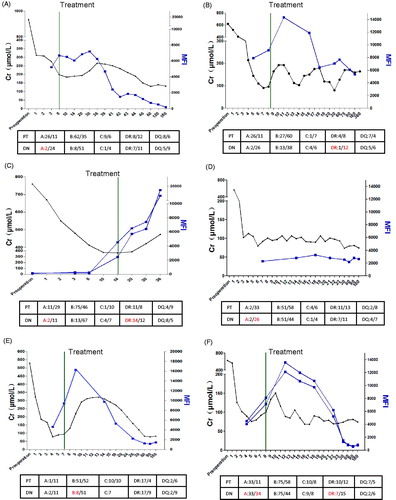
Table 7. Clinical data of DSA-positive patients.
Discussion
For a long time, the cellular immunity is considered to play a major role in the mechanism of transplant rejection. More and more evidences show that the HLA antibodies (humoral immune) play a main role in transplant rejection in recent years. The high PRA level during pre-transplantation indicates the high risk of rejection post-transplantation.Citation10 It was reported that 54% of patients with kidney transplant rejection were detected with HLA antibodies in serum.Citation11 In the early 1970s, Morris and Jeannette successively found that the antibody for the cause of the rejection was DSA.Citation12,Citation13 With the further increase of the antibody detection technology, Harmer et al. found that DSA was detected with flow cytometry technology in 95% of patients which showed rejection.Citation12 A large amount of specimen (826 cases of patients with organ rejection) research has shown that 96% of patients were detected DSA.Citation14
The current diagnosis of acute ABMR, as defined by the last Banff conference (2013), relies on the association of the following three criteria: first, histological evidence of acute tissue injury; second, evidence for the interaction of antibodies with the endothelium in the graft and third, serological evidence of HLA-specific antibodies or other DSAs at the time of biopsy.Citation15,Citation16 In our study, 239 patients were DSA−/NDSA−, 60 cases were DSA−/NDSA+, and 6 cases were DSA+/NDSA+. The AR occurrence of each group was 2.09%, 18.33% and 83.33%, respectively. All AR cases executed the grafts biopsy, in which five cases were found with high level of DSA in sera and diagnosed as ABMR. We also found that the AR patients with DSA+/NDSA+ recovered slowly and the recovery graft function was not as good as those with DSA-negative. That is because tissue injuries will occur through complement cascade activation and inflammatory cells in the microcirculation recruited via their Fcg receptors after interaction between circulating DSA and endothelium. This state may result in an acute graft dysfunction (acute ABMR) or be isolated without increase of serum creatinine (sub-clinical ABMR). In both cases, ABMR will lead to chronic ABMR and an accelerated graft senescence evidenced by the early onset of interstitial fibrosis and tubular atrophy (IFTA), cg and cv lesions.
Level of DSA is associated with the recipient immune status and can more accurately predict rejection reaction and the effect of treatment. Mohamed et al. found that MFI maximum value of DSA antibody raised above 500 within 1 week, which was the independent risk factor of ABMR.Citation17 Our findings also identified these phenomena. In our study, one of the DSA-positive patients had DSA MFI waved around 3000 and his creatinine value was also in the normal range. On the other hand, DSA MFI of five of other patients were raised more than 500 within 1 week, and all of them showed AR, which proved the importance of dynamic monitoring DSA at post-operative.
ABMR treatment measures such as elimination of DSA in circulation, inhibition of DSA resynthesis and application of strong immunosuppressive drugs, etc., were included.Citation18 In this study, four patients were given large doses of rabbit anti-thymocyte immune globulin (ATG) to treat AR, and then the serum creatinine were gradually restored and the DSA levels were gradually falling to a stable range after anti-rejection therapy. But one patient’s DSA level was not reduced obviously after ATG treatment. Also, her serum creatinine appeared gradually rising after a slight decline. After giving twice of plasma exchange, antibody levels (MFI) significantly decreased to 2000 gradually, and then followed with serum creatinine stable around 200 μmol/L. Taken together, ATG-induced therapy and plasma exchange treatment are two efficient ways to decrease DSA level.
It was reported that another cause of AR occurrence was the increased C1q levels.Citation19,Citation20 The level of C1q was closely related to the graft microvascular inflammatory and C4d deposition.Citation21 The 5-year survival rate of patients with DSA and C1q both positive was only 54%, which was far below the HLA antibodies-negative patients.Citation21 In our study, we also detected the C1q levels of six DSA-positive patients and found that three patients was C1q-positive. There were two cases occurred AR with DSA-positive but C1q negative. Such patients may be the result of graft injury by antibody-dependent cell-mediated cytotoxicity (ATCC), and the long-term graft survival of these kind recipients was closed to DSA-negative recipients as reported.Citation21
In brief, DSA persistent exist could affect the stability of the graft function, and the degree of DSA in the serum was associated with the graft function status. Dynamically, monitoring of DSA antibody level could effectively predict the AR occurrence. Rabbit anti-human thymocyte immunoglobulin-induced therapy and plasma exchange therapy could effectively reduce the DSA in sera. Long-term monitoring of DSA after organ transplantation could timely eliminate the DSA, predict the rejection reaction, and guide the immunosuppressive therapy. It plays an important role for effective prevention and in-time treatment of rejection.
Declaration of interest
This work was supported by grants from the Technology Research Project of Shaanxi Province (2012SF2-21, 2012K16-09-02), and the National Natural Science Foundation of China (81200545, 81270545). The authors declare that they have no competing interests.
References
- Gosset C, Lefaucheur C, Glotz D. New insights in antibody-mediated rejection. Curr Opin Nephrol Hypertens. 2014;23:597–604
- Klein C, DC. B. HLA and ABO sensitization and desensitization in renal transplantation. UpToDate; 2013; Available at: http://www.uptodate.com/contents/hla-and-abo-sensitization-and-desensitization-inrenal-transplantation. Accessed September 10, 2014
- Ren Q, Paramesh A, Yau CL, et al. Long-term outcome of highly sensitized African American patients transplanted with deceased donor kidneys. Transpl Int. 2011;24:259–265
- Ginevri F, Nocera A, Comoli P, et al. Posttransplant de novo donor-specific hla antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant. 2012;12: 3355–3362
- Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399
- Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167
- Lachmann N, Terasaki PI, Budde K, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87:1505–1513
- Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol. 2011;72: 849–858
- Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–760
- Morris PJ, Mickey MR, Singal DP, Terasaki PI. Serotyping for homotransplantation. XXII. Specificity of cytotoxic antibodies developing after renal transplantation. Br Med J. 1969;22:758–759
- Jeannet M, Pinn VW, Flax MH, Winn HJ, Russell PS. Humoral antibodies in renal allotransplantation in man. N Engl J Med. 1970;282:111–117
- Harmer AW, Koffman CG, Heads AJ, Vaughan RW. Sensitization to HLA antigens occurs in 95% of primary renal transplant rejections. Transplant Proc. 1995;27:666–667
- El-Awar N, Terasaki P, Lazda V, Nikaein A, Manning C, Arnold AN. Almost all patients who are waiting for a regraft of a kidney transplant have anti-HLA antibodies. Transplant Proc. 2002;34:2531–2532
- Lee PC, Zhu L, Terasaki PI, Everly MJ. HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation. 2009;88:568–574
- Colvin RB. Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056
- Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14: 272–283
- Mohamed MA, Muth B, Vidyasagar V, et al. Post-transplant DSA monitoring may predict antibody-mediated rejection in sensitized kidney transplant recipients. Clin Transpl. 2011:389–394
- Fehr T, Gaspert A. Antibody-mediated kidney allograft rejection: Therapeutic options and their experimental rationale. Transpl Int. 2012;25:623–632
- Freitas MC, Rebellato LM, Ozawa M, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95:1113–1119
- Thammanichanond D, Mongkolsuk T, Rattanasiri S, et al. Significance of C1q-fixing donor-specific antibodies after kidney transplantation. Transplant Proc. 2014;46:368–371
- Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215–1226

