Abstract
Urotensin II (U-II) was thought to be one of the mediators of primary renal sodium retention due to effects on renal sodium excretion. For this purpose, the relationship between U-II and overhydration was investigated. A total of 107 patients were enrolled in the study. According to body compositor monitor analysis, fluid overload up to 1.1 L, was considered normohydration. Patients were divided according to hydration status; overhydrate (n = 42) and normohydrate (n = 65) were studied in both groups. Pulse waveform velocity propagation for arterial stiffness and blood pressure analysis and echocardiographic left ventricular and left atrial indices were performed with known fluid overload-related parameters. U-II levels were measured by using Human ELISA kit. In overhydrated group, U-II levels were significantly lower. All parameters (blood pressure, arterial stiffness parameters, echocardiographic data, age, gender, diabetes, U-II, hemoglobin) correlated with overhydration, were determined by linear regression model (method = enter), when considered together, U-II was found to be an independent predictor from other conventional overhydration-related parameters. Male sex, left ventricular mass index, left atrial volume index, hemoglobin value were found to be independent predictors for overhydration. Considering the association of low U-II levels with adverse cardiovascular events and its role in sodium retention, we think that low U-II levels can be accepted as a potential therapeutic target in patients with hypervolemic cardio-renal syndrome.
Introduction
Urotensin II (U-II) is a potent vasoactive peptide ligand, initially isolated from the Goby fish. There are many homologous species for U-II, including humans. The kidney is a major source for U-II production.Citation1 U-II is mostly detected in a number of kidney cell types, including epithelial cells of tubules and ducts with greatest intensity in the distal convoluted tubules and the smooth muscle cells and endothelium of arteries.Citation2 Additionally, subtotally nephrectomized rats demonstrated significant increases in U-II expression in the kidneys,Citation3 which potentially implicates this peptide in the pathogenesis of progressive renal disease. Continuous infusion of U-II into the renal artery of anesthetized rats caused nitric oxide-dependent increases in GFR, urinary water excretion, and urinary sodium excretion.Citation5 This effect may be possibly explained by reduced renal functions and fluid overload association. In a model of arteriovenous fistula induced high-output heart failure, incremental injection of U-II caused mean arterial pressure lowering, both in failing heart and in healthy controls.Citation6 The net effect of U-II infusion on renal functions primarily depends on the underlying pathology: glomerular filtration rate tends to increase in models with heart failure, whereas GFR was decreased in models with no heart failure after U-II infusion. Our study population consisted of patients with either complicated or uncomplicated forms of hypertension and diabetes. We think that the presence of such complications may affect U-II levels and also its relations with volume status in these patients.
Primary and secondary renal sodium retention produces hypervolemia. In hemodialysis patients, excessive fluid intake and inadequate ultrafiltration are the basis of positive fluid balance. In predialysis patients, overhydration may occur as a result of cardio-renal syndrome which was characterized by renin angiotensin system (RAS) overactivity, and may result in decreased U-II activity according to our opinion. But in this group of patients, overhydration mechanism has not been elucidated sufficiently. Relationship between low U-II and cardiac malfunction was demonstrated in various clinical circumstances such as myocardial infarction.Citation4 Decreased production of U-II by insufficient renal parenchyma may contribute to hypervolemic state in patients with cardio-renal syndrome who are not yet on dialysis. As U-II is the most potent cardio-stimulant agent known today, subtle decreased myocardial performance in these patients may also contribute to this hypervolemia. Our goal is determined by endogenous mechanisms of fluid homeostasis in predialysis patients, and to examine the relationship between levels of U-II and conventional fluid overload-related parameters such as left ventricular hypertrophy, increase of pulse wave velocity (Pwv), blood pressure, diabetes mellitus, etc. and U-II is to determine whether or not it is a mediator for positive fluid balance.
Methods
Patient selection
This observational study was conducted at Antalya Training and Research Hospital. Our integrated chronic kidney disease (CKD) program included patients with CKD stages 3–4. CKD was staged according to KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines,Citation7 and eGFR was calculated using the four-variable MDRD (Modification of Diet in Renal Disease) study equation.Citation8 Exclusion criteria were as follows: any acute or chronic inflammatory state within 3 months, hospitalization related to dialysis within 3 months, diseases that lead to local fluid accumulation and edema, active malignancy, current use of diuretics (within 24 h), current use of any medication with the potential to influence body fluid composition, malnutrition, pregnancy, presence of a cardiac pacemaker or defibrillator, or the amputation of any extremity. All of the patients provided informed consent. This study was approved by the Institutional Review Board of the Antalya Education and Research Hospital.
Urotensin-II measures
Blood was taken in the morning on an empty stomach, for serum levels of U-II. Samples were taken into tubes containing EDTA and were centrifuged at 3000 rpm for 20 min. The serum obtained was stored at −80 °C. Determination of serum levels of U-II, Human U-II ELISA kit (cat. No: CK-E90124, Hangzhou Eastbiopharm Co. Ltd, Yile road, Hangzhou, China) was used. It was diluted with the standard solution to the level of standard 40 pg/mL U-II. Only stop solution was added to blank well (the empty box). For example, 40 µL of sample is added in an empty box, then 10 µL streptavidin-II antibody and 50 µL poly-HRP solution was added, which were incubated for 60 min at 37 °C. After washing five times with washing solution, chromogen A and solution B were added, which were incubated for 10 min at 37 °C. A total of 50 µL stop solution was added. Then, 15 min after adding stop solution, a zero value at a wavelength of 450 nm was measured from black well. Thus, through the optical density values that are obtained by the linear regression equation, U-II levels were measured.
Body composition monitor
A BCM (Fresenius Medical Care, Bad Homburg, Germany) was used to determine the extracellular and total body water. The BCM measures the impedance spectroscopy at 50 different frequencies between 5 kHz and 1 MHz. The BCM was validated intensively against all available gold-standard methods.Citation9 Electrodes were attached to one hand and one foot at the ipsilateral side, after the patient had been in recumbent position for at least 5 min. Extracellular water (ECW), intracellular water (ICW) and total body water (TBW) were determined from the measured impedance data according to the model of Moissl et al.Citation10
Echocardiography
Echo was performed on the subjects at rest in the left lateral decubitus position with synchronized electrocardiography by two experienced cardiologists who were blinded to the clinical data with a commercially available system (Philips iE33, Bothell, WA) equipped with a broadband S5-1 transducer (frequency transmitted: 1.7 MHz; received: 3.4 MHz). Standard techniques were used to obtain M-mode, 2D, and Doppler measurement in accordance with American Society of Echocardiography guidelines.Citation11
Left ventricular mass index (LVMI) was calculated from end-diastolic M-mode or 2D-guided measurements of the ventricular septum, LV internal diameter, and posterior wall thickness, according to the method of Devereux.Citation12 Teicholz Formula was applied to M-mode images resulting in left ventricular ejection fraction, end-diastolic volume and end-systolic volume [End-diastolic volume = 7/(2.4 + end-diastolic diameter) × (end-diastolic diameter)],Citation13 [end-systolic volume = 7/(2.4 + end-systolic diameter) × (end-systolic diameter)].Citation13
The maximal LA volume was measured from the apical four-chamber view by using the modified Simpson method in end-systole before mitral valve opening.Citation14 The left atrial volume index (LAVI) was obtained for all patients by dividing the LA volume by the body surface area. LV end-systolic and end-diastolic volumes along with the LVEF were calculated by the biplane Simpson’s method from apical four- and two-chamber views.
Assessment of pulse wave velocity
For the Pwv and central blood pressure analysis, Mobil-O-Graph® 24-h PWA monitor (I.E.M. Industrielle Entwicklung Medizintechnik und Vertriebsgesellschaft mbH Cockerillstraβe Stolberg, Rheinland, Germany) has been used. Built-in combining algorithms in the device were used for central aortic and brachial blood pressure measurements with combining pulse wave analysis. We used baPWv for the assessment of arterial stiffness. Augmentation index (AIx), pulse pressure (PP), and increased ankle brachial index (ABI) are also indices of arterial stiffness. AIx has traditionally been considered to represent increased wave reflection,Citation15 while more recently increased AIx has been attributed to a reduction in the reservoir function—compliance properties of the aorta and other elastic arteries.
Statistical analysis
Statistical results of baseline characteristics of all participants were stratified by fluid overload with cut-points 1.1 L. Categorical variables were expressed as percentages. The means of continuous variables were compared using Student's t-test for independent samples or the Mann–Whitney U-test depending on the distribution of data. Linear regression models were also used to evaluate the association of fluid overload with other body composition parameters, U-II levels, echocardiographic results and pulse wave analysis data. Statistical analyses were conducted using SPSS 17.0 for Windows (SPSS Inc., IBM software, Chicago, IL). Statistical significance was set at two-sided p value < 0.05.
Results
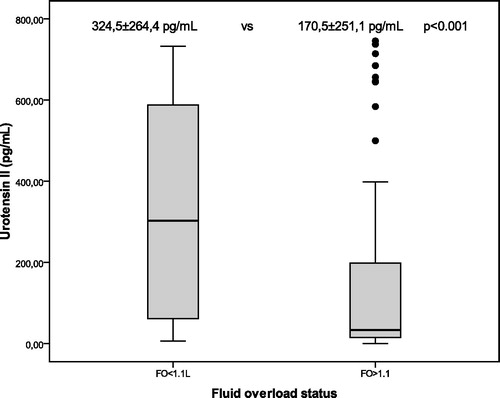
In , overhydration limit values for patients at BCM is divided into two groups as over and less than 1.1 L; in terms U-II (170.5 ± 251.1 pg/mL vs. 324.5 ± 264.4 pg/mL, p < 0.001, ), LVMI (p = <0.001), the difference between the two groups reached high statistical significance. Pwv (p = 0.024), systolic blood pressure (p = 0.37), pulse pressure (p = 0.005) were also higher in the group of overhydrated. Hemoglobin levels were significantly lower in hypervolemic group (p = 0.019). In non-diabetic CKD patients, U-II levels median 207.8 (2.2–745.1) pg/mL and in diabetic CKD group 57.1 (7.2–737.55) pg/mL were found (p = 0.3). Median levels of U-II, although in diabetic CKD patients were lower than non-diabetic CKD group but were not able to reach statistical significance potentially because of the influence from extreme values.
Table 1. Baseline characteristics of overhydrated and normohydrated patients.
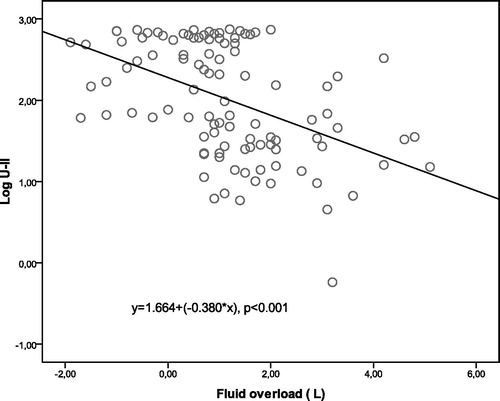
Analyzing , in all patient groups, between U-II, extracellular fluid and overhydration indicators, negative correlation is observed (). Positive relationship between the level of U-II obesity and insulin resistance are known.Citation20 In our study, a positive correlation (r = 0.204, p = 0.037) between the level of U-II and adipose tissue mass index seem to support these findings. A negative correlation is observed between U- II and LVMI (r = −0.176, p = 0.042). This case is consistent with negative correlation of U-II with the overhydration. There were no compliance in data obtained by the analysis of arterial stiffness and U-II. U-II and systolic blood pressure (r = −0.184, p = 0.034), pulse pressure (r = −0.204, p = 0.015), mean arterial pressure (r = −0.184, p = 0.032), central systolic pressure (r = −0.186, p = 0.031) was negatively correlated, significantly. All parameters (blood pressure, arterial stiffness parameters, echocardiographic data, age, gender, diabetes, U-II, hemoglobin, albumin) to be known correlated with overhydration, which were determined by linear regression model (enter method), when considered together, U-II (β = 0.243, p = 0.001) in showing overhydration was found to be an independent predictor from other parameters. Male sex (β = −0.267, p = 0.019), LVMI (β = 0.281, p = 0.015), LAVI (β = 0.312, p = 0.009), hemoglobin value (β = −0.249, p = 0.031) were found to be independent predictors for overhydration (Model R2 = 0.561).
Table 2. Urotensin II and body compartments correlation analysis.
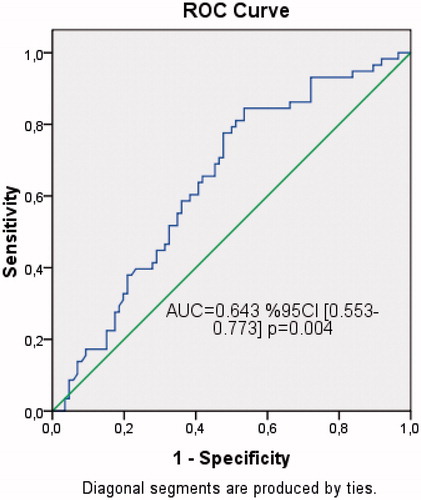
In ROC analysis (), U-II levels more than 35.4 pg/mL was predicted normohydration with 84% sensitivity and 46% specificity.
Discussion
Regulation of body fluid volume is determined by cardiac output (CO) and systemic arterial resistance.Citation16 Increased activation of the RAS with CKD, associated with hypertension, diabetes and ischemic cardiac dysfunction; as a result, pathophysiologic process, forming a positive fluid balance is activated. As GFR decreases by worsening CKD, blood pressure rises and is more difficult to control due to aberrant activation of the RAS and sympathic nervous system (SNS).Citation17 Overhydration, the resulting hyper inflammation, is one of the results of biochemical processes. The increase in inflammatory reaction, the effect is well known in CKD progression. Therefore, overhydration is one of the results of the deterioration in renal function process.Citation18
This is accompanied by reduced renal parenchymal mass and partial loss of normal regulatory functions, such as blood pressure regulation, erythropoiesis, and other vascular-protective processes. A high prevalence of events therefore occurs in this patient population.Citation19
In contrast to patients undergoing hemodialysis, in pre-dialysis chronic renal failure patients, hypervolemia etiology is multi-factorial. Balance between ultrafiltration during the dialysis and exogenous hydration creates fluid homeostasis of dialysis patients. However, in patients without dialysis, there are insufficient data regarding the pathogenesis of overhydration. Primary sodium retention, increased inflammation, cardiac dysfunction, increased activation of the renin-angiotensin system, hypoalbuminemia, is the primary mechanism that may be responsible for overhydration. Some studies reported that although elevation of U-II levels can be observed in various clinical diseases, low levels of U-II may be a predictor of poor outcome in these clinical situation.Citation20 This finding, the increase in level of the UII, suggests a compensatory response. Overhydration, in a study as determined the last point of entry into dialysis, shown to be an independent risk factor, over hydrated patients, in terms of cardio-renal damage parameters are thought to be at high risk. People with impaired renal function had plasma U-II levels significantly higher than subjects with normal renal function, the highest levels being observed in patients on dialysis.Citation21 It is not possible that whether the higher levels are a simple consequence of renal failure or can play a role in the pathogenesis of this pathologic condition. In our study determination of lower level in the group of overhydrated is compatible with the relationship of low levels of U-II with poor clinical status. UII can exert a direct effect upon myocardial function by increasing contractility in both atrial and ventricular tissue.Citation22 Decreased levels of UII may result in an increase in renal water and sodium retention due to decreased cardiac contractility and increased neurohormonal activation. Reduction in the number of functional nephron and increase in primary renal sodium retention occurs, may be the direct effect of the decrease in U-II is also possible. The negative correlation detected between fluid overload and plasma U-II levels supports this assertion. Analyzing overhydrated group, poor cardiovascular markers such as levels of blood pressure, Pwv index, LVMI and LAVI are significantly higher. Therefore, in this patient group, cardio-renal worse interaction is more intense. CKD is included at this stage, and a number of conditions associated with renal function decline, such as anemia, secondary hyperparathyroidism, or accumulation of atherogenic substances, become new CVD risk factors and accelerate vascular disease.
Conclusion
This study demonstrated that U-II activity is altered by pathophysiology of the underlying disease. In our study, population with cardio-renal syndrome, coexisting atherosclerosis and inadequate renal parenchyma may be associated with lower U-II levels. Low U-II levels may be a potential therapeutic target for such hypervolemic patients. We think that in hypervolemic patients with preserved renal functions, cardiac and renal effects of U-II levels should be investigated further.
Limitations
Our study is an observational, small-sized study. The association of U-II levels with natriuretic peptides was not evaluated in our study group.
Declaration of interest
This research is funded by Turkish Government Ministry of Health, Antalya Training and Research Hospital research committee. The authors report no conflicts of interest regarding the content herein.
References
- Charles CJ, Rademaker MT, Richards AM, et al. Urotensin II: Evidence for cardiac, hepatic and renal production. Peptides. 2005;26:2211–2214
- Shenouda A, Douglas SA, Ohlstein EH, et al. Localization of urotensin-II immunoreactivity in normal human kidneys and renal carcinoma. J Histochem Cytochem. 2002;50:885–889
- Mori N, Hirose T, Nakayama T, et al. Increased expression of urotensin II-related peptide and its receptor in kidney with hypertension or renal failure. Peptides. 2009;30:400–448
- Magdalena B, Michał H, Fryderyk P, et al. Is plasma urotensin II concentration an indicator of myocardial damage in patients with acute coronary syndrome? Arch Med Sci. 2012;8:449–454
- Zhang AY, Chen YF, Zhang DX, et al. Urotensin II is a nitric oxide-dependent vasodilator and natriuretic peptide in the rat kidney. Am J Physiol Renal Physiol. 2003;285:792–798
- Ovcharenko E, Abbasi Z, Rubinstein I, et al. Renal effects of human urotensin-II in rats with experimental congestive heart failure. Nephrol Dial Transplant. 2006;21:1205–1211
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470
- Wabel P, Chamney P, Moissl U, et al. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27:75–80
- Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921–933
- Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE Consensus Statement on Methodology and Indications Endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313
- Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension. 1987;9:19–26
- Teichholz LE, Kreulen T, Herman MV, et al. Problems in echocardiographic volume determinations: Echocardiographic–angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;12:1440–1463
- O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658
- Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585
- Bongartz LG, Cramer MJ, Doevendans PA, et al. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26:11–17
- Tsai YC, Tsai JC, Chen SC, et al. Association of fluid overload with kidney disease progression in advanced CKD: Prospective cohort study. Am J Kidney Dis. 2014;63:68–75
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305
- Khan SQ, Bhandari SS, Quinn P, Davies JE, Ng LL. Urotensin II is raised in acute myocardial infarction and low levels predict risk of adverse clinical outcome in humans. Int J Cardiol. 2007;117:323–328
- Totsune K, Takahashi K, Arihara Z, et al. Role of urotensin II in patients on dialysis. Letter to the Editor. Lancet. 2001;358:810–811
- Russell F, Molenaar P, O’Brien D. Cardiostimulant effects of urotensin II in human heart in vitro. Br J Pharmacol. 2001;132:5–9