Abstract
Recent studies have reported that remote organs are affected by renal ischemia reperfusion (IR). The present study investigates the role of vitamin E on the liver damage after renal IR. First, male mice were subjected to three groups (n = 9): 1) sham-operated, (2) renal IR (45 min ischemia), (3) renal IR + Vitamin E (150 mg/kg trough feeding tube for 28 d). After 24 h of reperfusion, animal were anesthetized for sample collections. Liver tissues malondialdehyde (MDA) increased and total glutathione (GSH) concentration decreased in the IR group compared to the sham group. Vitamin E consumption diminished the IR-induced increase in plasma AST and ALT. In addition, Vitamin E inhibited the IR-induced decrease in GSH activity and diminished IR-induced increase in MDA concentration. These findings showed that vitamin E consumption partly inhibited the IR-induced liver damage.
Introduction
Acute kidney injury (AKI) is a major clinical problem that occurs in some of the hospitalized patients especially in intensive care units. Renal ischemia reperfusion (IR) is one of the most important causative mechanisms of AKI and is associated with various clinical settings including shock, sepsis, kidney transplantation, vascular surgery, and elective urological operations.Citation1,Citation2
Studies have identified a close relationship between renal injury and other organ system failures. High mortality rate during AKI is partly due to remote organ manifestation. In previous studies, it has been demonstrated that severe ischemic AKI induces inflammation and functional changes in the brain.Citation1 In addition, AKI induces functional and transcriptional changes in the lung.Citation2
Cellular or humoral factors are considered to be the reasons of remote organ damage, but their exact pathophysiological mechanisms are not completely recognized.Citation3,Citation4
Recent studies have suggested cross-talk between the liver and kidneys. It has been demonstrated that renal IR induces oxidative stress in liver causing its dysfunction.Citation5
During renal IR, liver functional indices such as blood aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were elevated. It has been reported that renal IR increased myeloperoxidase activities and decreased superoxide dismutase, catalase and hepatic glutathione (GSH) levels in liver.Citation6,Citation7
Vitamin E (a-tocopherol) as the most important lipid soluble antioxidant has a major protective role against oxidative stress. Vitamin E acts mainly as a chain-breaking antioxidant lipid peroxidation of unsaturated fatty acids in the cell membrane and scavenges ROS (reactive oxygen species) and lipid peroxyl radicals.Citation8
You-Lin et al. reported Vitamin E decreases glomerulosclerosis and diminishes oxidative stress in the nephrectomized rat.Citation9 In addition, vitamin E diminished alcohol-induced changes in mice liver and protects against heavy metals-induced liver injury.Citation10,Citation11
This study was designed to investigate the protective effects of vitamin E on liver damage, as a remote organ injury, after renal IR. The present research is being carried out to highlight the possible useful effects of the usage of vitamin E as an antioxidant vitamin as complementary therapy in AKI patients in order to improve the side-effects of various clinical settings including kidney transplantation, vascular surgery, and elective urological operations and lower the morbidity induced by AKI.
Materials and methods
Male mice were purchased from Pasteur Institute (Tehran, Iran). They (weight = 25–30 g) were maintained at room temperature (22 ± 2 °C) in a 12:12-h light–dark cycle and with free access to standard diet and water. Animal were randomly assigned to three groups (n = 9): (1) Sham-operated, (2) renal IR (45 min ischemia), (3) renal IR + Vitamin E (150 mg/kg trough feeding tube for 28 d).
An established model of renal IR injury in mice was used.Citation6 Briefly, mice were anesthetized with intraperitoneal pentobarbital sodium (60 mg/kg). Systolic blood pressure was measured by the tail-cuff method connected to a pneumatic transducer using a PowerLab/4 sp data acquisition. Then a midline incision was made and the renal pedicles were bluntly dissected and occluded with non-traumatic vascular clips (Biemer-Clip, Aesculap, Tuttlingen, Germany) for 45 min. After ischemia, clamps were removed gently and the kidneys were observed for a further 5 min to ensure reflow process. Then, the incision was closed in two layers with a 4-0 silk suture. The animals were then returned to their cages and allowed to recover. During the period of renal ischemia, the animals were covered with plastic wrap to prevent evaporation. In addition, animals were kept well hydrated with warm sterile saline and were maintained at a constant body temperature (∼37 °C) on a heating pad. Sham-operated mice underwent surgical procedure identical to those of IR mice except that clamps were not applied. At the end of the reperfusion period (24 h), the animals were anesthetized. Blood samples were obtained from heart. The abdomen was opened immediately and liver tissues were collected and were snap frozen.
Biochemical assay
Plasma concentrations of blood urea nitrogen (BUN), plasma creatinine and liver function indices – ALT and AST – were evaluated by colorimetric methods using commercially available kits.
Liver oxidative stress indices
Malondialdehyde (MDA) level was evaluated in liver tissue by its reaction with thiobarbituric acid according to the Esterbauer and Cheeseman method.Citation12 Liver GSH was assayed according to the Griffith method 5, 5′-Dithiobis 2-nitrobenzoic acid was used as a chromogen and the absorbance of the reduced chromogen was measured at 412 nm.Citation13
Statistics
Results are expressed as means ± S.E.M. The statistical significance was determined by using one-way analysis of variance followed by Tukey’s post-hoc test. Results with a p < 0.05 were considered significant.
Results
Forty-five minutes ischemia followed by 24 h of reperfusion significantly increased plasma BUN and creatinine levels in IR group compared with sham group ().
Table 1. Plasma BUN, Cr, AST and ALT concentrations.
AST and ALT increased significantly in plasma after renal IR compared with sham group. Vitamin E diminished the IR-induced increase in plasma AST and ALT ().
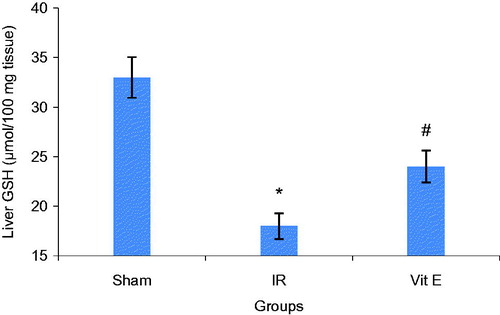
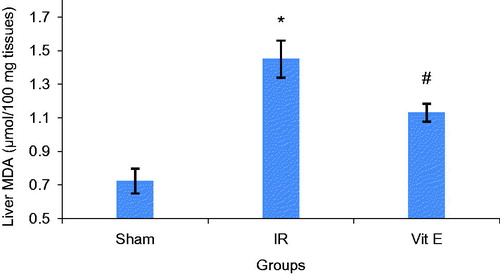
Decline in GSH activity and a marked increase of MDA in liver tissues were observed after renal IR compared to Sham group. Vitamin E partly inhibited the IR-induced decrease in GSH activity. Besides, Vitamin E moderately diminished IR-induced increase in MDA concentration in liver tissues ( and ).
Discussion
Renal IR damage is one of the most common causes of AKI and occurs in many clinical situations, such as transplantation, partial nephrectomy, sepsis, hydronephrosis, or elective urological operations. AKI still continues to be associated with a high mortality rate, particularly when associated with multiple organ failure. Cardiac, liver, brain and pulmonary injury have been documented after renal IR.Citation1,Citation14 Liver and kidneys are essential regulators of body homeostasis and are involved in the excretion of the waste products of metabolism and exogenous drugs.
In the present study, the changes in hepatic function and antioxidant status were evaluated after 45 min bilateral renal ischemia followed by 24-h reperfusion. As expected, renal IR caused a reduction in renal function as demonstrated by increased BUN and Cr levels in plasma.
The significant increase in AST and ALT suggests liver functional injury in IR group. It has been suggested that kidneys and liver have cross-talk during AKI. Our previous study has shown that IR causes hepatic detrimental functional changes as demonstrated by increased liver function indices, AST and ALT.Citation6,Citation7 Golab et al. showed that renal IR increase ALT, AST and LDH (lactate dehydrogenase).Citation5
The reduction in liver tissues GSH concentration and raise in liver tissues MDA concentration suggested that ROS mediated the biomolecular alterations.
Total glutathione (GSH), an important antioxidant in tissues and MDA, an end product of membrane lipid peroxidation, are two important indices that show oxidant-antioxidant balance in tissues.
It is worth mentioning that the role of inflammation has been documented in some organs dysfunction after AKI. One of the possible mechanisms involved in this failure might be due to ROS production. Inflammatory responses are activated during the process of IR injury, resulting in the production of inflammatory cytokines, such as tumor necrosis factor-alpha, IL-6, IL-10 and IL-17a.Citation15,Citation16
Some studies showed that the expression of spermine/spermidine-N1-acetyl transferase, an important enzyme in polyamine catabolic pathway, is upregulated in hepatic or kidney reperfusion injury and caused increased formation of ROS.Citation5,Citation16
Our findings showed that vitamin E administration diminished liver functional injury. Plasma AST and ALT increased after Vitamin E consumption in contrast to sham group. In addition, to some extent, vitamin E significantly inhibited the IR-induced decrease in GSH activity and IR-induced increase in MDA concentration in liver tissues.
It has been demonstrated by Al-Attar et al. in 2011 that vitamin E as an antioxidant diminished heavy metals-induced liver injury. They demonstrated vitamin E decreases heavy metals-induced elevation of ALT, AST and alkaline phosphatase.Citation11
In 2010, Kaur et al. reported that Vitamin E can decrease the toxic effects of alcohol and can be used as an important therapeutic factor for alcohol-induced oxidative injury in liver. They showed that Vitamin E inhibited alcohol-induced GSH elevation and Superoxide dismutase reduction.Citation10
Our findings clearly demonstrate that renal ischemia causes detrimental changes in liver, as a remote organ, partly due to oxidative stress. Then, care should be taken to keep other organs during renal ischemia, in particular during renal surgery. Vitamin E consumption partly inhibits this injury. Vitamin E, as a lipid soluble and chain-breaking antioxidant, is a best potent antioxidant because of easy, effective and safe dietary administration in a large range of concentrations. It is a very important contributor to non-enzymatic defense against lipid peroxidation and a well-known free radical scavenger.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19(7):1360–1370
- Hassoun HT, Grigoryev DN, Lie ML, et al. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol-Renal Physiol. 2007;293(1):F30–F40
- Kadkhodaee M, Khastar H, Seifi B, Najafi A, Delavari F. Renal oxidative injury after leukocyte transfer from ischemia-reperfusion-induced kidney damage in Balb/c mice. Acta Physiologica Hungarica. 2013;100(1):99–106
- Rabb H, Daniels F, O'Donnell M, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol-Renal Physiol. 2000;279(3):F525–F531
- Golab F, Kadkhodaee M, Zahmatkesh M, et al. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int. 2009;75(8):783–792
- Khastar H, Kadkhodaee M, Reza Sadeghipour H, et al. Liver oxidative stress after renal ischemia-reperfusion injury is leukocyte dependent in inbred mice. Iran J Basic Med Sci. 2011;14(6):534
- Khastar H, Kadkhodaee M, Sadeghipour HR, et al. Leukocyte involvement in renal reperfusion-induced liver damage. Renal Fail. 2011;33(1):79–83
- Abd-El-Fattah AA, El-Sawalhi MM, Rashed ER, El-Ghazaly MA. Possible role of vitamin E, coenzyme Q10 and rutin in protection against cerebral ischemia/reperfusion injury in irradiated rats. Int J Radiat Biol. 2010;86(12):1070–1078
- Tain Y-L, Freshour G, Dikalova A, Griendling K, Baylis C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am J Physiol-Renal Physiol. 2007;292(5):F1404–F1410
- Kaur J, Shalini S, Bansal M. Influence of vitamin E on alcohol-induced changes in antioxidant defenses in mice liver. Toxicol Mech Meth. 2010;20(2):82–89
- Al-Attar AM. Vitamin E attenuates liver injury induced by exposure to lead, mercury, cadmium and copper in albino mice. Saudi J Biol Sci. 2011;18(4):395–401
- Esterbauer H, Cheeseman KH. [42] Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Method Enzymol. 1990;186:407–421
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–212
- Kelly K, ed. Acute renal failure: Much more than a kidney disease. Semin in Nephrol. Elsevier; 2006;26(2):105--113
- Park SW, Chen SW, Kim M, et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Investig. 2010;91(1):63–84
- Kadkhodaee M, Golab F, Zahmatkesh M, et al. Effects of different periods of renal ischemia on liver as a remote organ. World J Gastroenterol. 2009;15(9):1113