Abstract
Hemorrhagic complications of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis are life-threatening conditions. Of these, alveolar hemorrhage is the most common and well-described hemorrhagic complication and is a prominent component of the pulmonary-renal syndrome. The clinical presentation of alveolar hemorrhage is highly variable ranging from mild to serious disease that may require respiratory support. Less attention has been directed at hemorrhagic complications that have been reported in other organs and which may be as severe or more severe with regard to morbidity and mortality. For example, among the most serious non-pulmonary complications are those related to cerebral and gastrointestinal hemorrhage. Cerebral hemorrhage is the clearly the most critical issue with a persistent high mortality rate. The prognosis of gastrointestinal hemorrhage, once an ominous complication has improved with medical and surgical intervention. Not to be dismissed are the consequences of hemorrhagic issues related to skin, soft tissue or muscle involvement. Muscle hematomas, while rare, also accompanied by significant morbidity.
Introduction
Anti-neutrophil cytoplasmic autoantibody (ANCA) disease comprises a group of autoimmune disorders including granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilia granulomatosis with polyangiitis (EGPA),Citation1 characterized by necrotizing small-vessel vasculitis with autoantibodies directed against neutrophil cytoplasmic constituents, in particular proteinase 3 (PR3) and myeloperoxidase (MPO). Focal necrotizing change is a common pathologic feature in the affected vessels.Citation2 Crescentic glomerulonephritis is a very common pathologic condition that can potentially progress to permanent renal dysfunction. Other complications such as pulmonary hemorrhage occur frequently,Citation3 whereas cerebral, gastrointestinal and other hemorrhagic complications are much less common but may be associated with significant morbidity and even mortality.
Detailed characteristics of patients with ANCA disease and associated hemorrhagic complications have been limited to a few individual case reports and case series. We conducted a systematic review to ascertain the epidemiology, clinical features, short-term and long-term outcomes of patients with hemorrhagic complications of ANCA disease and present the results against a background of an exceptional case of GPA manifesting as PR3-ANCA positive crescentic glomerulonephritis with near fatal alveolar hemorrhage and complicated by gastrointestinal bleeding and rectus muscle hematoma.
Case report
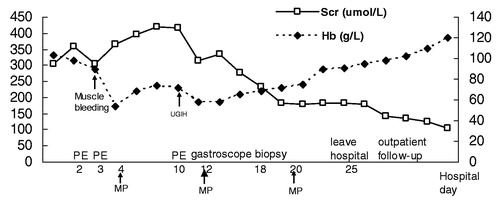
A previously healthy 67-year-old female was admitted to the China–Japan Friendship Hospital with constitutional symptoms of 5 weeks duration and a 3-d history of blood-tinged sputum. On admission, she was febrile with the most prominent physical findings consisting of obvious edema of her lower limbs with inspiratory crackles over her middle and lower right lung fields. Subsequent radiographic studies showed large areas of ground glass opacity consistent with alveolar hemorrhage were seen on chest films () and confirmed with thoracic CT scan (). Her serum creatinine concentration (Scr) was 283 μmol/L (3.2 mg/dL). Urinalysis showed the presence of microscopic hematuria and moderate proteinuria. Indirect immunofluorescence revealed cytoplasmic ANCA (c-ANCA) at a titer of 1:100 with anti-PR3 ANCA specificity by ELISA (340 U/L). She was diagnosed with an ANCA-related disease. Treatment with antibiotics and plasmapheresis was instituted. The clinical course is shown in . After the second plasmapheresis, she suddenly complained of abdominal pain and swelling in the right rectus muscle region accompanied by hypotension. Her hemoglobin (Hb) level dropped from 8.9 to 4.2 g/dL in the subsequent hours. Abdominal CT showed an extensive, irregular low-density mass in the right muscle (). On hospital day 5, she was started on methylprednisolone pulse therapy, 500 mg/d given intravenously for 3 d. This was followed by oral prednisolone (1 mg/kg/d) and intravenous cyclophosphamide (0.5 g/m2). This resulted in the cessation of hemoptysis resolution of the abnormal radiographic findings. However, she developed tinnitus and bloody nasal discharge and diagnosed as frontal sinuses by the CT scan. During this time, her Scr continued to increase and a third plasmapheresis was performed. On day 10, massive hematochezia occurred, with a drop in Hb from 8.3 to 5.2 g/dL. Endoscopy showed two duodenal ulcers approximately 2 cm in diameter (). Biopsies of these lesions showed inflammatory cells and fibrosis to the laminapropia. She received another MP pulse (500 mg/d × 3) along with transfusions and supportive therapy and gradually improved.
Figure 1. Chest X-ray (A) and CT (B) revealed large areas of ground glass opacity in the middle and lower lung fields. Abdominal CT (C and D) showed an extensive, irregular low-density mass in the right rectus muscle (white arrow). Gastrointestinal (GI) endoscopy demonstrated two duodenal ulcers around 2 cm diameter. Some of the mucosa between the ulcers is reddish and edematous. The border of the ulcer is unclear and covered with white coating (E). Another ulcer is square and clears (F). Renal biopsy showed two large fibrocellular crescent (black arrow) and a small cellular crescent (black thin arrow) in the renal glomeruli (G and H). Protein cast were presented with lymphomonocyte interstitial infiltration around glomeruli (H). Periodic acid-Schiff stain, 200×.
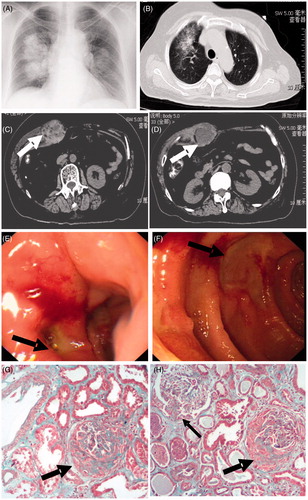
Figure 2. Clinical course of the patient. Scr, serum creatinine; Hb, hemoglobin; UGIH, upper gastrointestinal hemorrhage; PE, plasma exchange; MP, methylprednisolone.
Tissue obtained on percutaneous renal biopsy showed fibrocellular crescentic glomerulonephritis () consistent with a diagnosis of GPA. A third MP pulse (500 mg/d × 3) was given. Cyclophosphamide was continued monthly for total of six doses, followed by azathioprine (2 mg/kg/d). On day 25, the patient was discharged with improving Hb and Scr. During the 18-month follow-up, her Hb and PR3-ANCA were normal. The Scr had decreased to 96 μmol/L (1.1 mg/dL). Her drug therapy consisted of prednisone 10 mg on alternate days, and azathioprine 100 mg per day.
Discussion
The various forms of vasculitis are generally categorized by the size of the affected blood vessel. ANCA-associated vasculitis is one of several entities marked by inflammatory infiltrates of small vessels that occur in response to autoantibody deposition. Hemorrhagic complications occur most often with involvement of the renal and pulmonary vasculature. However, other tissues and organs may be affected as a result of increased permeability of vascular walls due to inflammation or occlusive disease consequent on thrombus formation.Citation4 At times, other complicating features such as the co-existence of severe hypertension or the consequences and/or side effects of treatment protocols are implicated in the etiology of the hemorrhage. The present case highlights the interaction of these factors in that the patient had life-threatening pulmonary hemorrhage as a result of the systemic vasculitis, gastrointestinal bleeding with inflammation and fibrosis of the laminapropia of the small intestine and a large muscle hematoma perhaps related to heparin therapy.
Alveolar hemorrhage is an important pulmonary manifestation of ANCA disease, with presentations ranging from mild to serious disease often requiring respiratory support. Indeed, severe, diffuse alveolar hemorrhage is the most common vasculitic cause of early death.Citation5 Alveolar hemorrhage can occur in the context of various systemic disorders or present as an isolated event.Citation6 The prevalence of alveolar hemorrhage in ANCA disease ranges from 10% to 45%.Citation7–11 A systematic review of a total of nine studies performed between 1985 and 2012 described the clinical features of alveolar hemorrhage.Citation12 The individual studies varied from 5 to 80 patients in size with a total of 207 patients. Hemoptysis and dyspnea were common but non-specific features. Although chest radiography is usually abnormal, high-resolution computerized tomographic scanning is a more sensitive diagnostic tool. Fiberoptic bronchoscopy and bronchoalveolar lavage are useful when a superimposed pulmonary infection is suspected.Citation12 Severe alveolar hemorrhage requiring ventilation occurs in 5% of patients with ANCA disease.Citation8 Despite increased understanding of pathogenesis and newer therapeutic options, patients with ANCA disease accompanied by severe alveolar hemorrhage have a 5-year patient survival of 75%.Citation13 However, this number is reduced to 50% at 2 years if ventilation had been required.Citation14 In a retrospective study, Hruskova et al. reported on a cohort of 53 patients and found that severe alveolar hemorrhage was more commonly associated with PR3-ANCA (vs. MPO-ANCA) and strongly correlated with renal vasculitis.Citation15 Mortality was higher in those requiring dialysis at entry and in patients aged >65 years, and tend to be higher in those requiring intubation.
Cerebrovascular involvement in ANCA disease is rare, occurring in from 0 to 3.6% of all cases.Citation16–18 Curiously, GPA patients with CNS involvement may have a high rate of ANCA negativity.Citation19 summarizes the clinical manifestations of intra-cerebral hemorrhage in patients with ANCA disease. The hemorrhagic neurological complications are thought to be secondary to weakening of blood vessel walls due to an inflammatory vasculitis, resulting in vessel rupture and subsequent bleeding.Citation18,Citation20 It is difficult to identify the pathogenesis of every case in the absence of an examination of vascular tissue obtained by biopsy or at the time of autopsy. By themselves, cerebral imaging methods may not be able to the specific findings of vasculitis that include irregularity of arterial walls or obstruction of arteries.Citation21 The clinical course of patients with cerebral hemorrhage is rapid progressive and the prognosis is poor. Less symptomatic lesions may respond to immunosuppressive therapy,Citation22 while more extensive and life-threatening lesions require emergency surgery in view of the associated high-mortality rate.Citation23–28 Thus, prompt diagnosis and treatment with immunosuppressive therapy and/or surgical intervention are essential. In addition to the vessel wall thickening due to the vasculitis itself, rupture of cerebral aneurysms, or complication of hypertension need to be considered.Citation29–31
Table 1. The clinical manifestations of intra-cerebral hemorrhage in patients with ANCA disease.
Gastrointestinal involvement is not rare with focal necrotizing arteriolitis having been detected by necropsy in 24% of the 56 GPA cases for whom data are available.Citation32 However, reports of significant clinical manifestations of gastrointestinal hemorrhage are rare. Since 1962, five cases with severe intestinal involvement were detected by emergency surgery due to perforation.Citation33–37 Two cases with MPA were reported to have duodenal ulcers.Citation38,Citation39 Pagnoux et al. presented 62 patients with medium- and small-sized vasculitis accompanied by gastrointestinal involvement. There was one patient with EGPA and two additional patients with MPA who developed bowel perforations and received surgery.Citation40 The detailed available information of those patients with gastrointestinal hemorrhage is listed in . It has been emphasized that vasculitis-related gastroduodenal ulcerations typically have an extensive and profound pattern.Citation41 The cause of ulcer formation in GPA is generally considered to be related to the vasculitis itself. Severe, occlusive damage often leads to ischemia that may result in ulceration and perforation whereas non-occlusive vascular disease may lead to vascular leakage resulting in edema and hemorrhage. Those patients often present with diarrhea or symptoms of bleeding.Citation42 The prognosis has dramatically improved, probably owing to better management of the more severely ill patients, with prompt surgical intervention when indicated, and the combined use of steroids and immunosuppressant.Citation40 Intestinal perforation has been reported where pathologic analysis of a surgically resected portion of the small intestine showed chronic suppurative inflammation in all layers of the ileal wall and mesentery.Citation43
Table 2. The clinical manifestations of gastrointestinal hemorrhage in patients with ANCA disease.
Although intra-muscular hematoma has been reported in 2% of patients with Henoch-Schönlein purpura,Citation44 reports of muscle hemorrhage in ANCA disease are rare with only two cases reported.Citation45,Citation46 One case with MPA was found to have femoral hemorrhage and another autopsy case of PR3-ANCA disease was complicated with rectus muscle hematoma. Commonly, GPA affects small vessels (capillaries, venules, or arterioles). However, since the report of aneurysm formation and free-vessel rupture of medium and large vessels in a patient with ANCA disease, the possibility that medium-sized vessels might be ruptured cannot be ruled out.Citation47 In our case, due to the absence of thrombocytopenia, disseminated intravascular coagulation, and trauma in the present case, GPA was considered to be the cause of the femoral hemorrhage. Although heparin was administered (5000 µ for plasmapheresis), the last dose was given 3 h before the occurrence of rectus hemorrhage. None-the-less, because heparin may accumulate in plasma due to the renal insufficiency and because its T1/2 is 1.5 h, the possibility that heparin contributed to the development of hemorrhage cannot be ruled out.
In conclusion, hemorrhagic complications of ANCA disease other than alveolar hemorrhage are uncommon but may be associated with significant morbidity and mortality. The patient in this report presented with rapidly progressive renal insufficiency, pulmonary hemorrhage, rectus muscle hematoma and duodenal ulcer in succession. It should be noted that multi-organ hemorrhages are serious complications and prompt diagnosis and treatment are required.
Acknowledgments
We are very grateful to National Natural Science Foundation of China and Youth Science and Technology Excellence Project of China-Japan Friendship Hospital for the financial assistance.
Declaration of interest
Financial assistance was provided by National Natural Science Foundation of China [grant number 81200535] and Youth Science and Technology Excellence Project of China-Japan Friendship Hospital [Grant number: 2014-QNYC-A-01]. The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article.
References
- Jennette J, Falk R, Bacon P, et al. Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2012;65:1–11
- Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636
- Jennette JC, Falk RJ. ANCA vasculitis: Microscopic polyangiitis, Wegener’s granulomatosis and Churg–Strauss syndrome. Pathol Case Rev. 2007;12:200–221
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J. 2006;82:483–488
- Casian A, Jayne D. Management of alveolar hemorrhage in lung vasculitides. Semin Respir Crit Care Med. 2011;32:335–345
- Krause ML, Cartin-Ceba R, Specks U, Peikert T. Update on diffuse alveolar hemorrhage and pulmonary vasculitis immunol allergy. Clin North Am. 2012;32:587–600
- Haworth SJ, Savage CO, Carr D, Hughes JM, Rees AJ. Pulmonary hemorrhage complicating Wegener’s granulomatosis and microscopic polyarteritis. Br Med J (Clin Res Ed). 1985;290:1775–1778
- ter Maaten JC, Franssen CF, Gans RO, van Schijndel RJ, Hoorntje SJ. Respiratory failure in ANCA-associated vasculitis. Chest. 1996;110:357–362
- Guillevin L, Durand-Gasselin B, Cevalos R, et al. Microscopic polyangiitis: Clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42:421–430
- Stone JH; Wegener’s Granulomatosis Etanercept Trial Research Group. Limited versus severe Wegener’s granulomatosis: Baseline data on patients in the Wegener’s granulomatosis etanercept trial. Arthritis Rheum. 2003;48:2299–2309
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232
- West S, Arulkumaran N, Ind PW, Pusey CD. Diffuse alveolar hemorrhage in ANCA-associated vasculitis. Intern Med. 2013;52:5–13
- Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–494
- Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: A 4-year, single-center experience. Am J Kidney Dis. 2002;39:42–47
- Hruskova Z, Casian AL, Konopasek P, et al. Long-term outcome of severe alveolar hemorrhage in ANCA-associated vasculitis: A retrospective cohort study. Scand J Rheumatol. 2013;42:211–214
- Deshpande PV, Gilbert R, Alton H, Milford DV. Microscopic polyarteritis with renal and cerebral involvement. Pediatr Nephrol. 2000;15:134–135
- Hattori N, Mori K, Misu K, Koike H, Ichimura M, Sobue H. Mortality and morbidity in peripheral neuropathy associated Churg –Strauss syndrome and microscopic polyangiitis. J Rheumatol. 2002;29:1408–1414
- Sasaki J, Hirato J, Nakazato Y, Tanaka T, Takeuchi H. An autopsy case of P-ANCA-positive microscopic polyangiitis with multiple cerebral hemorrhagic infarctions. No To Shinkei. 1998;50:56–60
- Reinhold-Keller E, de Groot K, Holl-Ulrich K, et al. Severe CNS manifestations as the clinical hallmark in generalized Wegener’s granulomatosis consistently negative for antineutrophil cytoplasmic antibodies (ANCA). A report of 3 cases and a review of the literature. Clin Exp Rheumatol. 2001;19:541–549
- Murphy JM, Gomez-Anson B, Gillard JH, et al. Wegener granulomatosis: MR imaging findings in brain and meninges. Radiology. 1999;213:794–799
- Yamashita Y. Cerebral vasculitis secondary to Wegener’s granulomatosis: Computed tomography and angiographic findings. J Comput Tomogr. 1986;10:115–120
- Takaoka H, Hashimoto A, Nogi S, et al. A case of granulomatosis with polyangiitis (Wegener’s granulomatosis) manifested with asymptomatic intracerebral hemorrhage. J Clin Immunol. 2013;36:58–61
- Tan J, Hussain A, Daiwajna R, et al. Microscopic polyangiitis complicated by intracerebral hemorrhage. Am J Case Rep. 2013;14:276–279
- Akkara Veetil BM, Schimmer BM. A case of limited systemic sclerosis with p-ANCA, complicated by multiple cerebral hemorrhages. Rheumatol Int. 2009;29:325–329
- Sassi SB, Ghorbel IB, Mizouni H, et al. Microscopic polyangiitis presenting with peripheral and central neurological manifestations. Neurol Sci. 2011;32:727–729
- Han S, Rehman HU, Jayaratne PS, Carty JE. Microscopic polyangiitis complicated by cerebral hemorrhage. Rheumatol Int. 2006;26:1057–1060
- Ito Y, Suzuki K, Yamazaki T, et al. ANCA-associated vasculitis (AAV) causing bilateral cerebral infarction and subsequent intracerebral hemorrhage without renal and respiratory dysfunction. J Neurol Sci. 2006;240:99–101
- Kimura H, Akutsu N, Shiomi R, et al. Subarachnoid hemorrhage caused by ruptured intracranial fusiform aneurysm associated with microscopic polyangiitis. Neurol Med Chir. 2012;52:495–498
- Takahashi JC, Sakai N, Iihara K, et al. Subarachnoid hemorrhage from a ruptured anterior cerebral artery aneurysm caused by polyarteritis nodosa. Case report. J Neurosurg. 2002;96:132–134
- Bouvard B, Lavigne C, Marc G, et al. Two consecutive episodes of intracerebral hemorrhage as the presenting feature of polyarteritis nodosa. Rev Med Interne. 2007;28:651–654
- Oomura M, Yamawaki T, Naritomi H, et al. Polyarteritis nodosa in association with subarachnoid hemorrhage. Intern Med. 2006;45:655–658
- Tokuda M, Kurata N, Daikuhara H, et al. Small intestinal perforation in Wegener’s granulomatosis. J Rhumatol. 1989;16:547–549
- Geraghty J, Mackay IR, Smith DC. Intestinal perforation in Wegener’s granulomatosis. Gut. 1986;27:450–451
- McNabb MR, Lennox MS, Wedzicha JA. Small intestinal perforation in Wegener’s granulomatosis. Postgrad Med J. 1982;58:123–125
- Storesund B, Gran JT, Koldingsnes W. Severe intestinal involvement in Wegener’s granulomatosis: Report of two cases and review of the literature. Br J Rheumatol. 1998;37:387–390
- Deniz K, Ozseker HS, Balas S, et al. Intestinal involvement in Wegener’s granulomatosis. J Gastrointestin Liver Dis. 2007;16:329–331
- Wakura D, Yoneda M, Kotani T, et al. A refractory case of MPO-ANCA-associated vasculitis presented with gastrointestinal ulcer, rapidly progressive glomerular nephritis and pulmonary multiple nodules. Nihon Rinsho Meneki Gakkai Kaishi. 2010;1:31–36
- Inaguma D, Kurata K, Ishihara S, et al. A case of MPO-ANCA-related vasculitis that recurred as gastrointestinal bleeding and presented difficulty in treatment. Nihon Jinzo Gakkai Shi. 1998;7:560–565
- Wilson RT, Dean PJ, Upshaw JD, Wruble LD. Endoscopic appearance of Wegener’s granulomatosis involving the colon. Gastrointest Endosc. 1987;33:388–389
- Pagnoux C, Mahr A, Cohen P, Guillevin L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides. Medicine. 2005;84:116–128
- Williams DH, Kratka CD, Bonafede JP, Katon RM. Polyarteritis nodosa of the gastrointestinal tract with endoscopically documented duodenal and jejunal ulceration. Gastrointest Endosc. 1992;38:501–503
- Geboes K, Dalle I. Vasculitis and the gastrointestinal tract. Acta Gastroenterol Belg. 2002;65:204–212
- Wang HL, Liu HT, Chen Q, Gao Y, Yu KJ. Henoch-Schonlein purpura with intestinal perforation and cerebral hemorrhage: A case report. World J Gastroenterol. 2013;19:2574–2577
- Abe M, Okada K, Maruyama N, et al. A case of femoral hemorrhage in a patient with microscopic polyangiitis with low levels of myeloperoxidase-antineutrophil cytoplasmic autoantibody. Clin Exp Nephrol. 2011;15:414–418
- Sakaguchi Y, Niihata K, Yasuda K, et al. Autopsy case of PR3-ANCA associated vasculitis complicated with rectus muscle hematoma. Nihon Jinzo Gakkai Shi. 2009;5:550–556
- Zhao MH, Sun QZ, Wang HY. Clinical and pathological characterization of patients with microscopic polyangiitis with medium artery involvement. Renal Fail. 2003;25:989–995
- Wolton EW. Giant cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J. 1958;2:265–270

