Abstract
Background: Pediatric studies are relatively scarce on the superiority of cystatin C over creatinine in estimation of glomerular filtration rate (GFR). This study measured cystatin C and serum creatinine levels, and compared GFR estimated from these two parameters in patients with chronic renal disease. Methods: This prospective, observational, controlled study included 166 patients aged 1–18 years diagnosed with stage I to III chronic renal disease, and 29 age- and sex-matched control subjects. In all patients, GFR was estimated via creatinine clearance, Schwartz formula, Zappitelli 1 and Zappitelli 2 formula and the results were compared using Bland–Altman analysis. Results: Patients and controls did not differ with regard to height, body weight, BMI, serum creatinine and serum cystatin levels, and Schwartz formula-based GFR (p > 0.05). There was a significant relationship between creatinine and cystatin C levels. However, although creatinine levels showed a significant association with age, height, and BMI, cystatin C levels showed no such association. ROC analysis showed that cystatin C performed better than creatinine in detecting low GFR. Conclusion: Cystatin C is a more sensitive and feasible indicator than creatinine for the diagnosis of stage I to III chronic renal disease.
Introduction
In children and adolescents with early stages of chronic kidney disease (CKD) and well-maintained fluid and electrolyte balance, urinalysis may be entirely normal. Therefore, a reduced glomerular filtration rate (GFR) may serve as the only clinical sign of kidney damage in these individuals.Citation1–3 In clinical practice, GFR is most frequently estimated using endogenous surrogate markers, of which serum creatinine remains the most widely used. Serum cystatin C is a relatively new endogenous marker that offers the advantages of constant production by all nucleated body cells and its almost total catabolism at the proximal tubule.Citation1–3
Cystatin C is a non-glycosylated 13-kDa basic protein that acts as a cysteine proteinase inhibitor, and is produced at a relatively constant rate. This constancy is apparently not influenced by the presence of inflammatory conditions, muscle mass, gender, body composition, or age (after 12 months).Citation4,Citation5 From a number of clinical studies of cystatin C,Citation6 including one in healthy children,Citation4 two key findings are evident. First, the concentration of serum cystatin C correlated better with directly measured values for GFR than did serum creatinine. Second, subtle decrements in GFR are more readily detected via serum cystatin C than by creatinine concentration.Citation6 Thus, while cystatin C is not a conventional marker of GFR, reciprocal values of serum cystatin C levels are reasonably well correlated with GFR in adultsCitation7 and children.Citation8 The rationale for combining serum creatinine and plasma cystatin C originated from the finding that both markers show differing sources of error. Serum creatinine levels are confounded by muscle mass and variable tubular secretion, whereas serum cystatin C has a different volume of distribution and may vary with the volume status.Citation9 Recently, many studies reported on the superiority of cystatin C over creatinine in the estimation of GFR.Citation10
This study aimed to identify cystatin C and serum creatinine levels, and to compare GFR estimated via these two parameters in CKD patients followed at the Pediatric Nephrology Unit of the Department of Pediatrics, Uludag University (Turkey).
Methods
This study included 166 Stage I to III chronic renal disease patients aged between 1 and 18 years who regularly attended follow-up visits at the Pediatric Nephrology Unit. The control group comprised 29 healthy, age- and sex-matched children with no known disease and no history of infection within the previous 4 weeks. Written confirmation was provided that the study protocol complied with the World Medical Association Declaration of Helsinki regarding the ethical conduct of research involving human subjects. The study was approved by the local ethical board and informed consent was obtained from the families of all patients.
The patients and their parents were carefully instructed in the collection procedure and also received written directions for 24-h urine collection. Parents were encouraged to practice the urine collection procedure with their child on the preceding day. For non-toilet-trained children, urine samples were collected using a sterile pediatric urine collection bag (Pediabag14-5501, Kendall Medical Products, Mansfield, MA). The bag was taped around the genital area, and the child’s regular diaper was worn over the bag. For toilet-trained children, urine samples were collected using standard urine collection cups. To obtain a minimum of 30 mL of urine, up to four voids were combined if necessary. All micturitions were stored immediately in preservative-free, Extran-cleaned (Extran, MA03; Merck, Darmstadt, Germany), 1-L plastic containers at temperatures <–12 °C before transfer to the research laboratories where samples were stored at ≤−20 °C until analyzed. Investigators explicitly asked parents about their child’s compliance and discussed the completeness of the urine collection in detail with the family after previous clinic visit. Urinary creatinine was measured according to Jaffe method (Fluitest Crea, Biocon Diagnostic, Ref 448, Lot G558, Denmark).
Patients’ age, sex, weight, and height were recorded. Blood samples were obtained and serum creatinine was measured by Fluitest kit (Biocon® Diagnostic Hecke 8, 34516 Vöhl/Marienhagen, Germany) based on Jaffe Kinetic Colorimetric method in an autoanalyzer (RA-XT). Serum samples were kept at −80 °C until later analysis for cystatin C levels with particle-enhanced immunonephelometry using an N latex cystatin C kit with a reference range 0.50–0.96 mg/L and a BN ProSpec plasma protein analyzer. All patients underwent GFR measurement using creatinine clearance method (GFR-creatinine). Creatinine clearance (in milliliters per minute per 1.73 m2) was calculated as follows: urine creatinine (in milligrams per deciliter) multiplied by urine volume per minute (in milliliters per minute) divided by plasma creatinine (in milligrams per deciliter), adjusted for body surface area.
The severity of CKD is denoted by a staging scheme that extends from occult kidney damage with well-preserved function (stage 1) to the level of kidney failure requiring renal replacement therapy (stage 5). Estimated GFR (GFR) is determined by creatinine, and the stages of CKD are defined as: Stage 1: ≥90 mL/min/1.73 m2, Stage 2: 60–89 mL/min/1.73 m2, Stage 3: 30–59 mL/min/1.73 m2, Stage 4: 15–29 mL/min/1.73 m2, and Stage 5: <15 mL/min/1.73 m2.
The study excluded patients with diabetes, hyperthyroidism, or hypothyroidism, those receiving treatment for malignancy, and individuals who underwent renal transplant or receiving renal replacement therapy (hemodialysis or peritoneal dialysis).
Body mass index (BMI) was calculated as weight in kilograms divided by the square of patient height in meters (kg/m2).
GFR was calculated from serum creatinine and cystatin C levels using relevant formulae. The Schwartz formulaCitation11 was used to estimate GFR from serum creatinine levels as follows:
Various formulae have been described for the estimation of GFR from cystatin C. In this study, we used the Zappitelli 1 and Zappitelli 2 formulae as followsCitation12:
Zappitelli 1 formula:
Zappitelli 2 formula:
Statistical analysis
Analyses were performed using SPSS version 13.0 and Med Calc software. Continuous variables were expressed as mean ± standard deviation (SD). For the comparisons between patients and controls, the Mann–Whitney U test was used, and the Kruskal–Wallis test was used for comparisons of more than two groups. Categorical data were compared using the Pearson chi-square test. Sensitivity, specificity, and cut-off values were estimated using ROC (receiving operating characteristic) analysis and agreement of GFR values was tested using Bland–Altman analysis. A p-value < 0.05 was considered statistically significant.
Results
The patient group comprised 166 children (80 boys), all of whom had Stage I to III chronic renal disease. The children had a mean age of 10.3 ± 0.4 (range 1–18) years. Mean BMI was 19.1 ± 0.3 (range 13–32) kg/m2. Serum creatinine level ranged from 0.27 mg/dL to 3 mg/dL (mean 0.69 ± 0.03 mg/dL). Serum cystatin C ranged from 0.17 mg/L to 3.20 mg/L (mean 0.76 ± 0.03 mg/L). Based on the Schwartz formula, mean GFR was 121.5 ± 22.6 (range 24–220) mL/min/1.73 m2. Mean GFR-creatinine clearance was 107.5 ± 46.3 (range 32–204) mL/min/1.73 m2.
The control group comprised 29 children, 13 of whom were boys. Mean age was 9.1 ± 0.7 (range 3–17) years. Mean BMI was 18.2 ± 0.7 (range 14–31) kg/m2. Serum creatinine level ranged from 0.42 mg/dL to 0.8 mg/dL (mean 0.55 ± 0.01 mg/dL). Serum cystatin C ranged from 0.25 mg/L to 0.89 mg/L (mean 0.60 ± 0.02 mg/L). In the control group, mean GFR based on the Schwartz formula was 132.3 ± 20.6 (range 92–284) mL/min/1.73 m2. Mean GFR-creatinine clearance was 134.6 ± 28.2 (range 98–262) mL/min/1.73 m2.
Patients and controls did not differ with regard to age, sex, height, body weight, BMI, serum creatinine and serum cystatin levels, or Schwartz formula-based GFR (p > 0.05). Although a significant association was found between creatinine levels and age, height, and BMI, no such relationship was present for cystatin C levels. A significant relationship was found between serum creatinine and cystatin C levels ( and ).
Table 1. The correlation between creatinine and cystatin C and age, height, BMI in patient group.
Table 2. The correlation between creatinine and cystatin C and age, height, BMI in control group.
GFR was estimated from creatinine and cystatin levels, both in patients and controls. While the three GFR values differed significantly in the patient group (p < 0.05), no such difference was evident in the controls (p > 0.05) (). In the patient group, mean GFR obtained via the Zappitelli 1 formula was higher than those from the Zappitelli 2 and Schwartz formulae. A comparison of GFR estimation methods with the index 24-h creatinine clearance method is presented in . The correlation coefficients (r2 values) for the Schwartz, Zappitelli1 and Zappitelli 2 equations were 0.64, 0.77 and 0,86 respectively (p < 0.05).
Table 3. GFR results in patients and controls.
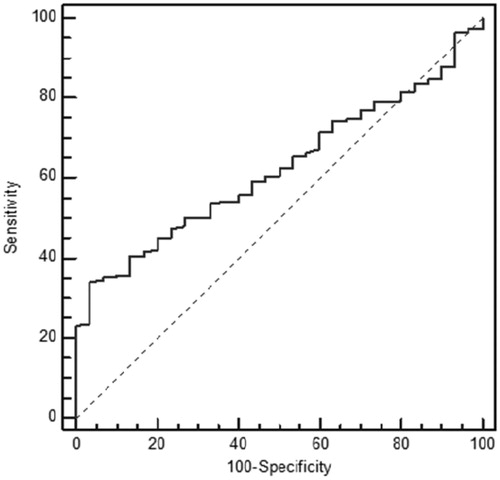
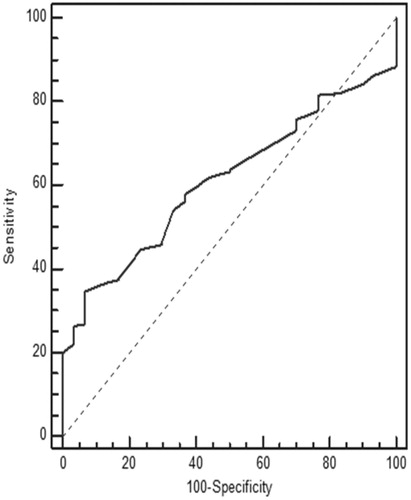
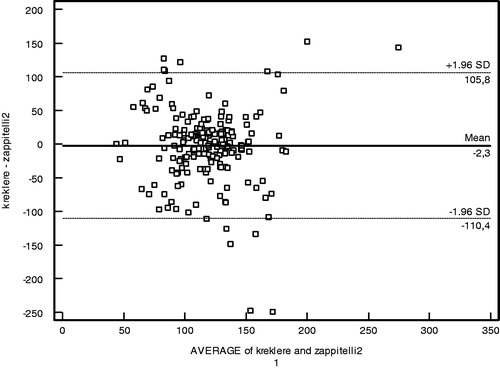
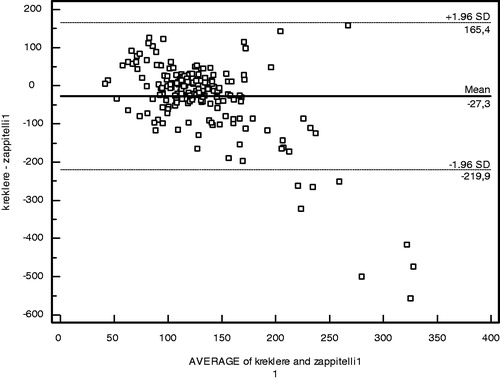
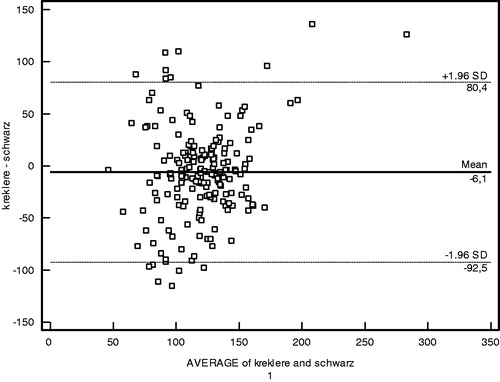
The performances of creatinine and cystatin in detecting low GFR (<90 mL/min/1.73 m2) were evaluated by receiver operating characteristic (ROC) analysis with Schwartz-derived GFR serving as the reference ( and ). The cut-off value for cystatin C was 0.62 mg/L with 70.83% sensitivity, 58.45% specificity, and area under the curve (AUC) of 0.63 ± 0.05 (p = 0.02) (). For creatinine, the cut-off value was 0.58 mg/dL with 66.67% sensitivity, 45.77% specificity, and AUC of 0.52 ± 0.06 (p = 0.74) (). Based on the ROC analysis, cystatin C showed better performance than creatinine in detecting low GFR. The agreement of the three GFR values with creatinine clearance was evaluated using Bland–Altman analysis. The best agreement was found between Zappitelli 2 formula-based GFR and creatinine clearance, which showed 3.1% deviation (). No significant relationship was found between creatinine clearance and GFR derived via the Zappitelli 1 or Schwartz formulae ( and ).
Discussion
The results show that, based on ROC analysis, cystatin C performed better than creatinine in detecting children with chronic renal disease. The agreement of the three GFR measurements with creatinine clearance was evaluated using Bland–Altman analysis. The Bland–Altman analysis is not a statistical test measured via a p-value; instead, it is a process used to assess agreement among methods of measurement. GFR estimated via the Zappitelli 2 formula showed the best agreement with creatinine clearance. Age, height, and BMI affected the estimation of GFR from creatinine levels; however, cystatin C was not affected by these parameters. Cystatin C had greater sensitivity than creatinine in detecting low GFR.Citation10,Citation13–16 GFR estimated from cystatin C provided more consistent results than that estimated from creatinine clearance using the Schwartz formula. Serum cystatin C has been examined in a number of patient populations, including healthy children,Citation17 renal transplant recipients,Citation18 and patients with glomerulonephritis or various renal diseases.Citation19–21 In many studies, serum cystatin C-derived GFR was equivalent to radioisotope (125-I iothalamate) determination of GFR and 24-h urine for creatinine clearance, and was superior to serum creatinine for monitoring renal impairment.Citation22 GFR based on the serum creatinine Schwartz formula showed poor performance across all GFR levels and in each of the Stage I to III CKD groups in our study. Our findings are in agreement with those of previous studies in addition to the investigation of serum cystatin C as a marker of GFR in patients with chronic renal disease.Citation19–21 Bacchetta et al. evaluated the external validity of several published bedside formulas based on plasma creatinine or serum cystatin C in comparison with the reference method in a French pediatric cross-sectional cohort of 252 patients.Citation23 Bacchetta et al. showed that most of the cystatin C-derived formulas for estimating GFR seem accurate in non-selected pediatric patients and also suggested that the cystatin C-derived formulas can be used reliably when reference methods for measuring the true GFR are not available or when the local adaptation of Schwartz formula has not been performed.Citation23 Children with the Stage I to III CKD were evaluated instead of non-selected children in our study when compared with Bacchetta et al. Creatinine suffers a high degree of inter-individual variability related to sex, age, and body composition. Previous studies reported elevated cystatin C levels in intensive care unit patients during the early phase of acute renal injury, and in association with severe liver disease, cardiac surgery, progressive renal nephropathy, and unilateral nephrectomy.Citation13–16 In this study, age, height, and BMI affected the estimation of GFR from creatinine levels; however, cystatin C was not affected by these parameters. Cystatin C had greater sensitivity than creatinine in detecting low GFR. GFR estimated from cystatin C provided more consistent results than GFR estimated from creatinine clearance using the Schwartz formula. Cystatin C is a helpful indicator with the potential to replace creatinine for the estimation of GFR in routine clinical practice.
The GFRs estimated from creatinine and cystatin C are both associated with sources of errors. The variation in the production rate can be estimated, and would appear to be greater for creatinine than cystatin C. Creatinine has a variable tubular secretion and reabsorption but low non-renal clearance. Cystatin C, on the other hand has greater non-renal clearance, which also appears to vary. The sources of error for estimating GFR from cystatin C and creatinine are distinctly different. Our study has shown that cystatin C had greater sensitivity than creatinine in detecting low GFR. Some studies have suggested that the serum concentration of cystatin C might be superior to serum creatinine in distinguishing normal from abnormal GFR.Citation24 However, because it is metabolized and not excreted, cystatin C cannot be used to measure GFR by standard urinary clearance techniques.Citation6 Other studies have shown that plasma cystatin C is slightly better than plasma creatinine in diagnosing renal insufficiency but is less sensitive than creatinine clearance.Citation25 Moreover, cystatin C levels may underestimate GFR in renal transplant patients.Citation25 More recent studies have shown that factors other than renal function, such as C-reactive protein (CRP) and smoking status, may influence serum cystatin C concentrations, so that caution must be used when interpreting serum cystatin C levels as a measure of renal function.Citation26 The findings of cystatin C in urine during glomerular and tubular injury also cast some doubt on the suitability of serum cystatin C to accurately estimate GFR.Citation27,Citation28
In large-scale epidemiological studies, collection of seven 24-h urine specimens is very difficult practically.Citation29 Many challenges continue to exist in relation to obtaining a urine sample from young and non-toilet trained children. Invasive methods need to be considered for assessment of creatinine clearance. It is difficult to take the accurate 24-h urine in young and non-toilet trained children, so 24-h urine could be collected by using urinary catheter in our patients.
Our study had some limitations. The first was the absence of GFR measurement by a gold standard such as 99mTc-DTPA scintigraphy to assess the relationship between cystatin C- or creatinine-based formulae. Second, we did not measure cystatin C in Stage IV and V CKD groups. Another limitation was that our control group was also relatively small compared with our study group.
In conclusion, Cystatin C may be a more sensitive and feasible indicator for the diagnosis of chronic renal disease since it is not affected by factors such as age, height, and BMI. Our results suggest that the use of cystatin C can strengthen the role of GFR in diagnosing patients with Stage I to III chronic renal disease. The results of this study support that serum cystatin C is a reliable marker for detecting GFR in patients with Stage I to III chronic renal disease.
Declaration of interest
The authors declare there is no conflict of interest in this article.
References
- Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. CKD: Common, harmful, and treatable–World Kidney Day 2007. Am J Kidney Dis. 2007;49:175–179
- Coresh J, Laterza OF, Price CP, Scott MG. Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047
- Finney H, Newman DJ, Thakkar H, Fell JM, Price CP. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000;82:71–75
- Uemura O, Ushijima K, Nagai T, et al. Reference serum cystatin C levels in Japanese children. Clin Exp Nephrol. 2010;14:453–456
- Dworkin LD. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2001;10:551–553
- Christensson A, Ekberg J, Grubb A, Ekberg H, Lindstrom V, Lilja H. Serum cystatin C is a more sensitive and more accurate marker of glomerular filtration rate than enzymatic measurements of creatinine in renal transplantation. Nephron Physiol. 2003;94:19–27
- Sharma AP, Yasin A, Garg AX, Filler G. Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clin J Am Soc Nephrol. 2011;6:1599–1608
- Huang SH, Filler G, Yasin A, Lindsay RM. Cystatin C reduction ratio depends on normalized blood liters Processed and Fluid Removal during Hemodialysis. Clin J Am Soc Nephrol. 2011;6:319–325
- Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122
- Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263
- Zappitelli M, Parvex P, Joseph L, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–230
- Kezama JJ, Kutsuwada K, Ataka K, Maruyama H, Gejyo F. Serum cystatin C reliably detects renal dysfunction in patients with various renal diseases. Nephron. 2002;91:13–20
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483
- Grubb AO. Cystatin C-properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–69
- Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C-A new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–881
- Filler G, Witt I, Priem F, Ehrich JHH, Jung K. Are cystatin C and beta2-microglobulin better markers than serum creatinine for prediction of a normal glomerular filtration rate in pediatric subjects? Clin Chem. 1997;43:1077–1078
- White C, Akbari A, Hussain N, et al. Estimating glomerular filtration rate in kidney transplantation: A comparison between serum creatinine and cystatin C-based methods. J Am Soc Nephrol. 2005;16:3763–3770
- Wasilewska A, Zoch-Zwierz W, Jadeszko I, et al. Assessment of serum cystatin C in children with congenital solitary kidney. Pediatr Nephrol. 2006;21:688–693
- Alvarez O, Zilleruelo G, Wright D, Montane B, Lopez-Mitnik G. Serum cystatin C levels in children with sickle cell disease. Pediatr Nephrol. 2006;21:533–537
- Bokenkamp A, Herget-Rosenthal S, Bokenkamp R. Cystatin C, kidney function and cardiovascular disease. Pediatr Nephrol. 2006;21:1223–1230
- Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221–226
- Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L. Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol. 2011;6:552–560
- Laterza OF, Price CP, Scott MG. Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707
- Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C serum concentrations underestimate glomerular filtration rate in renal transplant recipients. Clin Chem. 1999;45:1866–1868
- Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421
- Tkaczyk M, Nowicki M, Lukamowicz J. Increased cystatin C concentration in urine of nephrotic children. Pediatr Nephrol. 2004;19:1278–1280
- Uchida K, Gotoh A. Measurement of cystatin-C and creatinine in urine. Clin Chim Acta. 2002;323:121–128
- Liu K, Cooper R, Soltero I, Stamler J. Variability in 24-hour urine sodium excretion in children. Hypertension. 1979;1:631–636