Abstract
We have investigated the effects of ketamine-based and remifentanil-based anesthetic protocol on perioperative serum cystatin-C levels, and creatinine and/or cystatin-C-based eGFR equations in terms of acute kidney injury in coronary artery bypass graft (CABG) surgery. Using a simple randomization method (coin tossing), patients were divided into the two groups and not-blinded to the anesthetist. Remifentanil–midazolam–propofol or ketamine–midazolam–propofol-based anesthetic regimen was chosen. Different eGFR formulas using creatinine (MDRD, CKD-EPI, Cockrauft Gault); cystatin-C (eGFR1, eGFR2) or a combination of creatinine and cystatin-C (eGFR 3) were used to calculate estimated glomerular filtration rates (eGFRs). High-sensitive troponin T was used to determine if ketamine use in coronary surgery contributed to myocardial cell damage. Thirty-seven patients were included in the study (remifentanil group = 19, ketamine Group = 18). Urea, creatinine, cystatin-C levels were comparable between the groups in all the measurement times and also postoperative day 2 samples showed statistically higher results compared to baseline (p < 0.001). Effects of ketamine and remifentanil on renal functions were found similar. Creatinine and cystatin-C-based eGFR equations resulted similar in our study. Reversible stage 1 acute kidney injury (AKI) was observed on postoperative day 2 in seven patients from the remifentanil group and six patients from the ketamine group. Hs-troponin T was found to be higher in postoperative day 1 samples; there were no significant difference between the groups. Our results indicated that patients who have normal renal functions undergoing on-pump coronary bypass surgery, effects of ketamine and remifentanil on renal functions in terms of AKI were found to be similar.
Introduction
Acute kidney injury (AKI) occurring following on-pump heart surgery is a condition elevating multifactorial morbidity and mortality. AKI causes an increase in length of stay in both intensive care and hospital.Citation1 Several factors, such as low arterial blood pressure, toxins, metabolic factors, hypoxia-reoxygenation, neurohumoral activation and systemic inflammation play a role in AKI patho-physiology.Citation2 Exogenous factors inducing this pathology include cardiopulmonary bypass (CBP), hypothermia, transfusion, cross-clamp and CPB duration, complicated and combined surgeries, hemodilution, thromboemboli and artificial and medical heart supports. Among endogenous factors are advanced age, female gender, chronic obstructive pulmonary disease, diabetes mellitus, peripheral vascular disease, poor ventricle, emergent surgery and the individual’s basal kidney functions, antioxidant capacity and response to inflammation.Citation3,Citation4
Serum cystatin-C is a valuable predictive biomarker of AKI following CPB.Citation5,Citation6 It was claimed that the use of cystatin-C shortens time to diagnosis of AKI by 1–2 days in patients in critical conditions.Citation7 It was shown that ketamine reduces the systemic inflammatory response induced by CPB and it has anti-inflammatory properties.Citation8–10 Although the use of ketamine is considered as disadvantageous in cardiac surgery because it causes an increase in blood pressure, ketamine–midazolam–propofol combination balances the blood pressure. Hypotension that may occur with opioid combinations does not develop with ketamine, and thus organ perfusions may be protected without vasoactive medicine.
In this study, we investigated the possible positive effects of ketamine (used as a sole analgesic in patients undergoing elective coronary surgery), which was shown to have positive impacts on the immune system, and on renal functions. Our primary hypothesis is that ketamine decreases acute kidney dysfunction and injury, as measured by postoperative cystatin-C levels undergoing coronary surgery. We also hypothesized that whether a ketamine-based regimen puts coronary patients at extra risk of myocardial injury, as measured by high-sensitive cardiac troponin levels.
Materials and methods
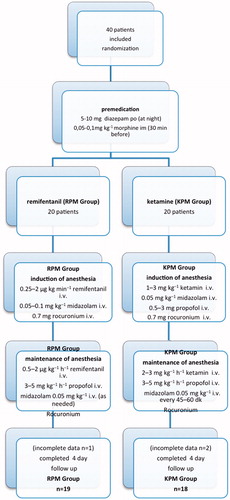
This study was approved by the Ethics Committee of Hospital (paperwork date/number: April 9th 2013/3979), had a clinical trial number of NCT02113150 and all patients were informed and gave written consent. Forty patients scheduled for elective CABG with cardiopulmonary bypass were enrolled in the study. We excluded patients undergoing emergency operation, combined operation, off-pump surgery, repeat surgery or valve surgery, patients presenting with chronic kidney disease or renal impairment, patients younger than 18 years old and neurological and/or psychiatric disturbances, patients who underwent angiography more than a week shortly before. Patients were scored using EuroSCORE II (Papworth Hospital, Cambridge, UK). Preoperative medications were continued until the morning of the operation. Standard premedication regimen was used in all patients (). Using a simple randomization method (coin tossing), patients were divided into the two groups and not-blinded to the anesthetist. Remifentanil–midazolam–propofol or ketamine–midazolam–propofol-based anesthetic regimen was chosen () for induction and maintenance. All patients in this study received propofol and midazolam in order to prevent ketamine-induced hallucinations. Rocuronium was used as a muscle relaxant. During the operation (except for CPB period) systolic arterial pressure and heart rate were maintained within ± 20% of baseline. Hemodynamic changes occurred during anesthesia (tachycardia = pulse > 100 beat/min, hypertension = systolic arterial pressure (SAP) > 160 mmHg, hypotension =SAP < 100 mmHg) were corrected using anesthetic medicine, cardiovascular medicine or fluids.
Monitoring included 5-lead electrocardiography (ECG), pulse oximetry, invasive artery and central venous access, urinary catheter, temperature probe, capnography and bispectral index (BIS). Routine surgical techniques and cardioprotection strategies were utilized in all patients. Patients had median sternotomy with harvesting saphenous vein and internal mammary artery. CPB was performed in the patients. Following the surgery, the patients were admitted to the intensive care unit (ICU) intubated, and weaned once they achieved proper breathing and hemodynamics. Blood samples were obtained before the induction of anesthesia (baseline), at the end of the operation and morning of postoperative days 1, 2 and 4.
Biochemistry analysis
All samples were centrifuged for 10 min at 1300 g; after the completion of clotting, they were stored at −20 °C until analysis. All of the analyses were performed on the same day. Cystatin-C levels were determined in a Roche Modular P analyzer (Roche Diagnostics, Germany) and high sensitive troponin t levels were analyzed using a Cobas E601 analyzer (Roche, Germany) with original reagents. Different eGFR formulas using creatinine (Modification of Diet in Renal Disease [MDRD], The Chronic Kidney Disease Epidemiology [CKD-EPI], Cockrauft Gault); cystatin-C (eGFR1, eGFR2) or a combination of creatinine and cystatin-C (eGFR 3) were used to calculate estimated glomerular filtration rates (eGFRs). Gold standard procedures for measurement of GFR (inulin, iohexol, 51Cr-EDTA, 99mTc-DTPA and 125I-iothalamate clearance) are time-consuming, have side effects for patient or expensive.Citation11,Citation12 Estimated GFR (eGFR) formulas have been developed and used in more than 80% of clinical laboratories in the United States because of the aforementioned problems.Citation13 The most common formulas used in the calculation of eGFR include MDRD and recently CKD-EPI equations.Citation14,Citation15 These equations were developed to determine eGFR based on not only serum creatinine but also demographic variables, such as age, gender and race.Citation16 It has been suggested that cystatin-C, which is used in eGFR estimations, gives more reliable results when used alone or with serum creatinine and other demographic variables.Citation17 Calculation of GFR by serum creatinine alone is affected by low- or high-muscle mass, diet and medications which have influence on tubular secretion. In vast majority of the studies with cystatin-C, it was reported that cystatin-C is a better marker for demonstrating GFR and its efficiency increases especially when used with keratinize.Citation17 Furthermore, cystatin-C responds more quickly to the decrease in GFR; cystatin-C starts to increase when GFR falls below 80 mL/min, but creatinine starts to increase when GFR falls below 40 mL/min.Citation18 For these reasons, cystatin-C is suggested to be more useful for demonstrating mild to moderate renal function disorders.Citation19 High-sensitive troponin T was used to determine if ketamine use in coronary surgery contributed to myocardial cell damage.
Statistical analysis
Data were analyzed using SPSS for Win 15 (SPSS/IBM, Chicago, IL). Descriptive statistics were presented as mean ± SD for normally distributed variables, median (min–max) for abnormally distributed variables and the number of case and percentage (%) for nominal variables. When it is normally distributed, the significance of the mean differences between times and groups was achieved by variance analysis test with repeated measurements. The significance of the median difference and significance of differences between in-group times were analyzed using Friedman’s test with Bonferroni correction. In case of a difference, multi-comparisons between times were evaluated using proper post-hoc tests. Intergroup means differences were evaluated using independent t-test, and the differences in medians were evaluated using Mann–Whitney test. Nominal variables were evaluated using Pearson’s Chi-square and Fisher’s exact tests. p < 0.05 was considered statistically significant. Reference values for cystatin-C have shown to be between 0.52 and 0.98 mg/L with a standard deviation of 0.19 mg/L.Citation20 Elevated serum cystatin-C was defined as ≥1.09 mg/L at the study of Muntner et al.Citation21 At least one SD elevation was assumed to be different among groups with regard to that paper.Citation21 Post-hoc power analysis revealed that this study has a power of 84% with these values. Power analysis was performed using the G*Power software (G*Power Version 3.1.5, Kiel, Germany).
Results
A total of 37 patients were studied (remifentanil group = 19, ketamine group = 18). Three patients were excluded due to missing data. Patient characteristics, preoperative medication, EuroSCORE II and cardiac profile data were similar between the groups ().
Table 1. Patient characteristics, preoperative medication, euroscore II and cardiac profile data.
During the intraoperative period, cross-clamp time, CPB time, the need for transfusion and 28-day mortality rates were found similar between groups (). During anesthesia period, SAP and heart rate (HR) were found to be higher in the ketamine group, which was not statistically significant.
Table 2. Intraoperative data, 28 day mortality.
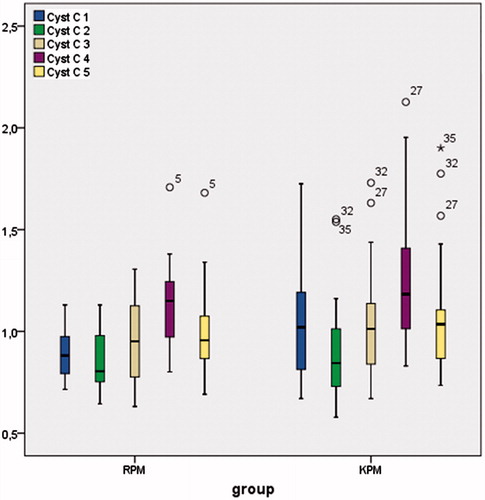
Urea (), creatinine (), and cystatin-C () levels were comparable between the groups in all of the timings and also postoperative day 2 samples showed statistically (except urea) higher results compared to baseline (p < 0.001). In the remifentanil group, baseline urea levels were 33 ± 13 mgdL − 1; postoperative second day levels were 44 ± 21 mgdL−1; in the ketamine group, baseline urea levels were 31 ± 17 mgdL−1; postoperative second day levels were 42 ± 21 mgdL−1. In the remifentanil group, baseline creatinine levels were 0.82 ± 0.18 mgdL−1; postoperative second day creatinine levels were 1.06 ± 0.33 mgdL−1; in the ketamine group baseline creatinine levels were 0.97 ± 0.36 mgdL−1; postoperative second day creatinine levels were 1.20 ± 0.55 mgdL−1. In the remifentanil group, baseline cystatin-C levels were 0.90 ± 0.13 mgL−1; postoperative second day cystatin-C levels were 1.12 ± 0.22 mgL−1; in the ketamine group, baseline cystatin-C levels were 1.05 ± 0.26 mgL−1; postoperative second day cystatin-C levels were 1.27 ± 0.36 mgdL−1.
Figure 2. Urea levels (mg dL−1) are presented as box plots for each group and time point. Note: For remifentanil group: urea 2–4 p < 0.01, urea 3–4 p < 0.05, urea 4–5 p < 0.001. For group KPM: urea 2–4 p < 0.05, urea 3–4 p < 0.05.
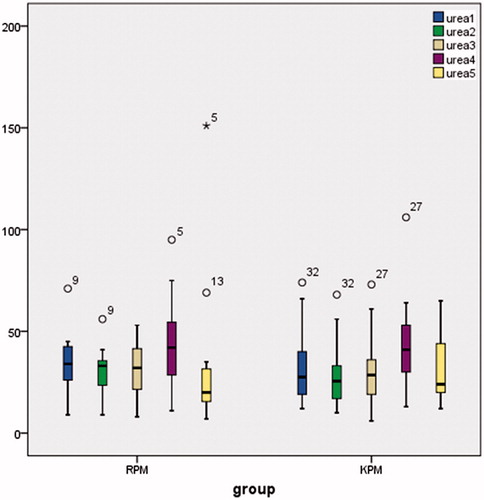
Figure 3. Creatinine levels (mg dL−1) are presented as box plots for each group and time point. Note: Both for groups Creatinine 1–4 p < 0.05, Creatinine 2–4 p < 0.001.
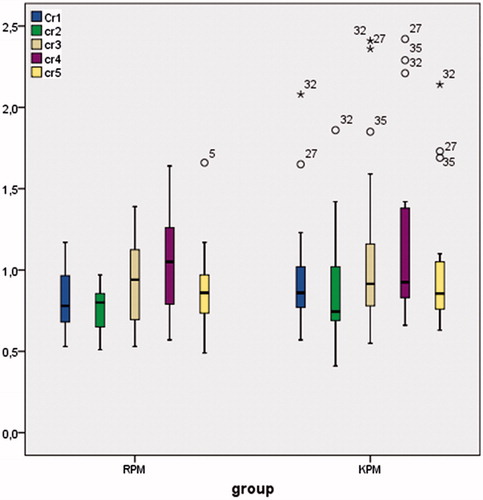
Figure 4. Cystatin-C levels (mg L−1) are presented as box plots for each group and time point. Note: For remifentanil group: Cystatin-C 1–4 p < 0.001, Cystatin-C 2–4 p < 0.001, Cystatin-C 3–4 p < 0.01. For ketamine group: Cystatin-C 1–4 p < 0.01, Cystatin-C 2–4 p < 0.001, Cystatin-C 3–4 p < 0.001, Cystatin-C 4–5 p < 0.01.
High sensitive (Hs)-troponin T was found to be higher in postoperative day 1 samples (p < 0.05); there were no significant differences between the remaining groups (). In the remifentanil group baseline Hs-troponin T level (mean ± SD) was 19 ± 15 ng/L; postoperative first day level was 662 ± 723 ng/L, in ketamine groups baseline Hs-troponin T level was 18 ± 21 ng/L; postoperative first day level was 575 ± 505 ng/L.
Figure 5. Hs-troponin T levels (ng/L) are presented as box plots for each group and time point. Note: Both for groups: Hs-troponin T 1–2 p < 0.001, Hs-troponin T 1–3 p < 0.001, Hs-troponin T 1–4 p < 0.001, Hs-troponin T 1–5 p < 0.05, Hs-troponin T 3–5 p < 0.001.
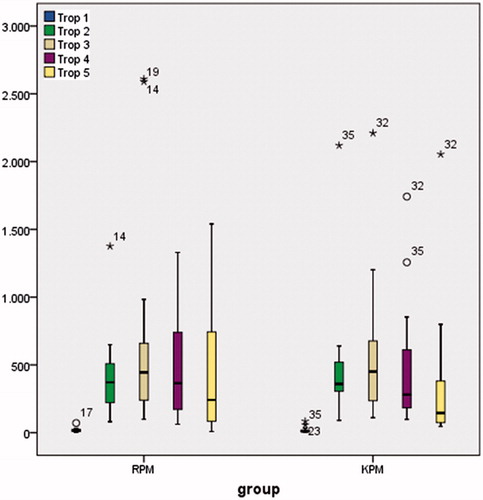
Different eGFR formulas using creatinine (MDRD, CKD-EPI, Cockrauft Gault); cystatin-C (eGFR1, eGFR2) or a combination of creatinine and cystatin-C (eGFR 3) were used to calculate eGFR. eGFR1, eGFR2, eGFR3, MDRD, CKD-EPI and Cockrauft Gault levels were similar between the groups and postoperative second day samples were statistically lower than baseline (p < 0.05).
Stage 1 AKI, which is known as a dysfunction, was observed in seven patients from the remifentanil group and six patients from the ketamine group on postoperative day 2. Creatinine values that increased by 0.3 g dL−1 or more compared to basal values on postoperative day 2 decreased back on postoperative day 4; none of the patients experienced stage 2 AKI ().
Table 3. Data of the patients with stage 1 AKI in postoperative day 2.
There was no mortality or morbidity after surgery in both the groups.
Discussion
In the present study, in patients with normal renal functions undergoing on-pump coronary bypass, the effects of use of ketamine and remifentanil as anesthetic agents on AKI development were compared by renal biomarkers. The effects of ketamine and remifentanil on renal functions were found similar. Cystatin-C and eGFR values varied in parallel with classical markers, urea and creatinine. After the surgery, GFR increased; urea, creatinine and cystatin-C decreased in both groups compared to basal values. On postoperative day 1, GFR started to decrease; urea, creatinine and cystatin-C started to increase. On postoperative day 2, after reaching the lowest GFR value, it increased on postoperative day 4, and the values were observed to be close to the basal values. In seven patients from the remifentanil group and in six patients from the ketamine group, reversible stage 1 AKI, which is known as a dysfunction, was observed on postoperative day 2. It was observed that ketamine use in coronary bypass surgery did not cause any cardiac adverse event.
According to the literature, in on-pump cardiac surgery, ketamine weakens the increase of CRP, IL-6 and IL-8, all of which are proinflammatory cytokines, and causes in increase in anti-inflammatory cytokine IL-10 and thus positively affects the functional outcome.Citation8,Citation9,Citation22 The inflammation period, which is an important risk factor for organ dysfunction following major surgeries, is known to be among AKI pathogeneses.Citation2,Citation3 The study was based on these premises and it was observed that 1–3 mgkg−1 ketamine infusion during surgery was not better than remifentanil infusion in terms of kidney functions. Since the study included only patients with normal basal renal functions, ketamine may not have created detectable effects on healthy kidneys. To identify the renal injury, if IL-18, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), urinary microalbumin, urinary N-acetyl-β-glucosaminidase (NAG) and similar markers, all of which increase at various periods during surgery, were used, more detailed physiopathological panel could have been obtained, but it would be a costly method.
It was previously reported in the literature that remifentanil weakens endocrine stress response and cell-mediated immune response, and shortens the ICU stay.Citation23,Citation24 This may be the reason why no difference between groups was observed. A follow-up study might include cardiac patients with similar renal dysfunctions to compare ketamine and remifentanil in terms of their immune protective effects determined by more elaborated markers. In the present study, racemic ketamine (R-(−)-ketamine) was used. In the literature, it was reported that isomeric S-(+)-ketamine had higher analgesic potential and fewer side effects and it was also reported that S-(+)-ketamine decreased the coronary adherence of polymorphonuclear neutrophils during postischemic period.Citation10 Renal effects of these two different ketamine isomers are also worth to investigate.
Due to the limited blood flow in coronary artery patients, the use of medications, such as ketamine, which increases myocardial oxygen demand, causes concerns. In the present study, high sensitive troponin T, which is a very valuable tool, was used to determine if R-ketamine use in coronary surgery contributed to myocardial cell damage. Hs-troponin T values increased similarly in both groups after the surgery and decreased in the following days, and no adverse cardiac event was observed. However, it should be noted that patients who are elective and hemodynamically stable were included in the study.
In the present study, cystatin-C and eGFR values varied in parallel with urea and creatinine, which are classical markers, and use of cystatin-C did not yield a quicker result. In the literature, it was reported that plasma use of cystatin-C yields faster results compared to creatinine in detecting renal injury.Citation5–7 On the other hand, it was also reported that there is a weak correlation between plasma cystatin-C and creatinine values in individuals with normal healthy renal functions.Citation25–27 It was also reported that effects of hemodilution during CPB may still are active in ICU and thus, eGFR values may be confusing.Citation28 In a multi-center study performed by TRIBE-AKI consortium, cystatin-C was found to be less sensitive compared to creatinine in terms of AKI detection.Citation29 In another study, it was reported that eGFR values estimated by combining creatinine and cystatin-C measurement were more accurate.Citation30 In the present study, cystatin-C, creatinine and all related eGFR calculations varied in the same way in all measurement points; therefore, none of them was shown to be better than the other.
According to the AKIN criteria, the minimum 0.3 mgdL−1 increase in creatinine value within 48 h is considered as stage 1 AKI which is known as a dysfunction.Citation1 In the present study, in seven patients from the remifentanil group and in six patients from the ketamine group, transient stage 1 AKI (dysfunction) was observed on postoperative day 2. Creatinine values that increased by 0.3 mgdL−1 or more compared to basal values on postoperative day 2 decreased back on postoperative day 4; none of the patients experienced stage 2 AKI. In the consortium study of Spahillari, post-cardiac surgery AKI detection time was reported as days 2 and 3; the increases in creatinine and cystatin-C on days 2 and 3 got back to normal values on day 4.Citation29 In the present study, creatinine and cystatin-C values that were increased on day 2 in both groups came close to the basal values on day 4. Since no measurement was performed on day 3, we just guess that there was a decreasing trend. Similarly, Abu-Omar reported that along with plasma creatinine and cystatin-C values, urinary NAG and urinary microalbumin/creatinine values reached peak levels on day 2 following cardiac surgery.Citation31 The reason for peak levels on postoperative day 2 may be that oral intake starts for patients discharged from ICU to the general ward and intravenous fluids were reduced. Even though oral intake is started on these patients, the intake is not as much as IV replacement. Furthermore, the detection of the cellular damage caused by inflammatory cascade using markers may be delayed.
Consequently, in patients with normal renal functions undergoing on-pump coronary bypass surgery, effects of ketamine and remifentanil on renal functions in terms of AKI were found to be similar. It was observed that the use of R(−)-ketamine in coronary bypass surgery did not cause a cardiac adverse event.
Declaration of interest
The authors report no conflicts of interest.
References
- Shaw A. Update on acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2012;143(3):676–681
- Bellomo R, Auriemma S, Fabbri A, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). Int J Artif Organs. 2008;31(2):166–178
- Gude D, Jha R. Acute kidney injury following cardiac surgery. Ann Card Anesth. 2012;15(4):279–286
- Parida S, Badhe AS. Cardiac surgery-associated acute kidney injury. J Anesth. 2013;27(3):433–446
- Shlipak MG, Coca SG, Wang Z, et al. TRIBE-AKI Consortium. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis. 2011;58(3):366–373
- Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–1122
- Larsson A, Malm J, Grubb A, et al. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64:25–30
- Dale O, Somogyi AA, Li Y, Sullivan T, Shavit Y. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg. 2012;115(4):934–943
- Welters ID, Feurer MK, Preiss V, et al. Continuous S-(+)-ketamine administration during elective coronary artery bypass graft surgery attenuates pro-inflammatory cytokine response during and after cardiopulmonary bypass. Br J Anaesth. 2011;106(2):172–179
- Neuhäuser C, Preiss V, Feurer MK, et al. Comparison of S-(+)-ketamine- with sufentanil-based anaesthesia for elective coronary artery bypass graft surgery: Effect on troponin T levels. Br J Anaesth. 2008;100(6):765–771
- Goldberg TH, Finkelstein MS. Difficulties in estimating glomerular filtration rate in the elderly. Arch Intern Med. 1987;147(8):1430–1433
- Feng JF, Qiu L, Zhang L, et al. Multicenter study of creatinine- and/or cystatin C-based equations for estimation of glomerular filtration rates in Chinese patients with chronic kidney disease. PLoS One. 2013;8(3):e57240
- Miller WG. Estimating glomerular filtration rate. Clin Chem Lab Med. 2009;47(9):1017–1019
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612
- Teo BW, Ng ZY, Li J, Saw S, Sethi S, Lee EJ. The choice of estimating equations for glomerular filtration rate significantly affects the prevalence of chronic kidney disease in a multi-ethnic population during health screening. Nephrology (Carlton). 2009;14(6):588–596
- Teo BW, Xu H, Wang D, et al. Estimating glomerular filtration rates by use of both cystatin C and standardized serum creatinine avoids ethnicity coefficients in Asian patients with chronic kidney disease. Clin Chem. 2012;58(2):450–457
- Laterza OF, Price CP, Scott MG. Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem. 2002;48(5):699–707
- Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34
- Croda-Todd MT, Soto-Montano XJ, Hernández-Cancino PA, Juárez-Aguilar E. Adult cystatin C reference intervals determined by nephelometric immunoassay. Clin Biochem. 2007;40(13–14):1084–1087
- Muntner P, Winston J, Uribarri J, Mann D, Fox CS. Overweight, obesity, and elevated serum cystatin C levels in adults in the United States. Am J Med. 2008;121(4):341–348
- Bartoc C, Frumento RJ, Jalbout M, Bennett-Guerrero E, Du E, Nishanian E. A randomized, double-blind, placebo-controlled study assessing the anti-inflammatory effects of ketamine in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2006;20(2):217–222
- Winterhalter M, Brandl K, Rahe-Meyer N, et al. Endocrine stress response and inflammatory activation during CABG surgery. A randomized trial comparing remifentanil infusion to intermittent fentanyl. Eur J Anaesthesiol. 2008;25(4):326–335
- Von Dossow V, Luetz A, Haas A, et al. Effects of remifentanil and fentanyl on the cell-mediated immune response in patients undergoing elective coronary artery bypass graft surgery. J Int Med Res. 2008;36(6):1235–1247
- Le Brıcon T, Thervet E, Benklakehal M, Bousquet B, Legendre C, Erlich D. Changes in plasma cystatin C after renal transplantation and acute rejection in adults. Clin Chem. 1999;45(12):2243–2249
- Plebani M, Dall’Amico R, Mussap M, et al. Is serum cystatin C a sensitive marker of glomerular filtration rate (GFR)? A preliminary study on renal transplant patients. Renal Failure. 1998;20:303–309
- Gökkuşu CA, Ozden TA, Gül H, Yildiz A. Relationship between plasma Cystatin C and creatinine in chronic renal diseases and Tx-transplant patients. Clin Biochem. 2004;37(2):94–97
- Svenmarker S, Häggmark S, Holmgren A, Näslund U. Serum markers are not reliable measures of renal function in conjunction with cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2011;12(5):713–717
- Spahillari A, Parikh CR, Sint K, et al. TRIBE-AKI Consortium. Serum cystatin C- versus creatinine-based definitions of acute kidney injury following cardiac surgery: A prospective cohort study. Am J Kidney Dis. 2012;60(6):922–929
- Ma YC, Zuo L, Chen JH, et al. Chinese eGFR Investigation Collaboration. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72(12):1535–1542
- Abu-Omar Y, Mussa S, Naik MJ, MacCarthy N, Standing S, Taggart DP. Evaluation of Cystatin C as a marker of renal injury following on-pump and off-pump coronary surgery. Eur J Cardiothorac Surg. 2005;27(5):893–898