Abstract
Introduction: The pathogenetic mechanisms underlying ischemia-reperfusion (I/R) injury involve oxidative stress, inflammation and apoptosis. Nesfatin-1, a novel peptide, has been reported to possess antioxidant, anti-inflammatory and anti-apoptic properties. The study was to examine the potential protective effects of nesfatin-1 on renal I/R injury. Materials and methods: I/R model was induced by placing a clamp across left renal artery for 45 min followed by 24 h reperfusion, along with a contralateral nephrectom. Twenty-four rats divided into three groups: sham-operated group, vehicle-treated I/R and nesfatin-1-treated I/R. Nesfatin-1 was intraperitoneally injected 30 min before renal ischemia. We harvested serum and kidneys at 24 h after reperfusion. Renal function and histological changes were assessed. Marker of oxidative stress and cells in kidney were also evaluated. Results: The animals with nesfatin-1 significantly improved renal functional and histologic lesions induced by I/R injury. The malondialdehyde (MDA) level decreased, whereas superoxide dismutase (SOD) and catalase (CAT) activities were significantly increased. Moreover, nesfatin-1-treated rats had a markedly decrease in apoptotic tubular cells, as well as a decrease in caspase-3 activity and an increase in the bcl-2/Bax ratio. Conclusions: This is the first evidence that nesfatin-1 treatment ameliorates acute renal I/R injury by suppressing oxidative stress and cell apoptosis. Therefore, it is promising as a potential therapeutic agent for renal IR injury.
Introduction
Renal ischemia followed by reperfusion, which causes severe oxidative injury to tissues and organs,Citation1,Citation2 still remains a serious problem in clinical procedures, such as hemorrhagic shock, organ procurement, hydronephrosis, vascular surgery, accidental or iatrogenic trauma, sepsis, partial nephrectomy and renal transplantation. Also when blood flow of the kidney is restored, additional reperfusion injury occurs. Increasing evidence indicates that renal ischemia-reperfusion (I/R) injury is an important cause of acute kidney injury (AKI). It contributes to the development of acute renal failure (ARF), which is associated with high mortality and morbidity in patients with transplanted kidney.Citation3
The mechanisms underlying IR injury are complex and incompletely understood, but oxidative stress, necrosis, cell apoptosis, ATP depletion and calcium dyshomeostasis undoubtedly contribute to the mechanisms of renal ischemia–reperfusion injury.Citation4–8 When renal I/R injury occurs, the increased generation of reactive oxygen species (ROS) in the reperfusion phase leads to lipid peroxidation, DNA mutation, as well as initiation of apoptotic and necrotic cascades, and ultimately leading to cell death.Citation9 Minimizing the adverse effects of IR injury would ameliorate outcomes in renal surgeries and decrease acute and chronic rejections in renal transplant recipients.
Nesfatin-1 is an 82-amino-terminal fragment discovered in the brain, which is derived from nucleobinding-2 (NUCB2), was first identified in 2006 by Oh and colleagues.Citation10 It has previously been reported that the administration of nesfatin-1 decreased food intake, induces a fear response and produces anxiety in rats.Citation10–13 In addition, Bonnet et al. found that neurons that produce nesfatin-1 are sensitive to peripheral inflammatory signals.Citation14 It was demonstrated that nesfatin-1 can cross the blood–brain barrier without saturation after systemic delivery in rats.Citation15,Citation16 Recently, it has also reported that nesfatin-1 possessed antioxidant, anti-inflammatory and anti-apoptotic properties, and protects the heart against I/R injury.Citation17–19 However, to the best of our knowledge, there have been no articles to report the protective effects of nesfatin-1 against renal I/R injury.
In light of the above findings, the aim of the present study was to investigate the potential antioxidant and anti-apoptotic effects of intraperitoneal nesfatin-1 on renal I/R injury in a murine model.
Materials and methods
Animals
Male Wistar rats (200–250 g) were purchased from the Hubei Provincial Academy of Preventive Medicine. The experimental protocol used in this study was approved by the Animal Ethics Review Committee of Wuhan University, and the procedures were carried out accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animals were housed in individual cages in a temperature-controlled (22–24 °C) room with alternating 12-h light/dark cycles with the relative humidity (65–70%) and acclimatized for a week before the study. The animals were allowed free access to food and water until 12 h prior to surgery when food (but not water) was removed.
Experimental group and protocol
The rats (n = 24) were randomly divided into three groups (each consisting of eight rats):
I. Sham group: rats pretreated with 0.9% NaCl solution, 1 mL/kg, intraperitoneally (ip) given 30 min before the sham operation;
II. I/R group: rats pretreated with 0.9% NaCl solution, 1 mL/kg, ip given 30 min before ischemia for 45 min followed by reperfusion for 24 h;
III. I/R + nesfatin-1 group: rats pretreated with nesfatin-1 (10 μg/kg, Alexis Biochemicals, San Diego, CA) ip given 30 min before ischemia for 45 min followed by reperfusion for 24 h. This dose was selected based on the results of previous studies and a preliminary experiment.Citation18
All the groups received the same volume of injection. Animal preparation was performed as previously described.Citation20 Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg). The abdominal region was shaved and sterilized with povidone iodine solution. A midline incision was made to expose renal pedicle, followed by a right nephrectomy. The left renal hilus was occluded using a non-traumatic microvascular clamp for 45 min to effect complete cessation of renal blood flow, and the intestine was replaced into the abdominal cavity. After 45 min, the clamps were removed to restoration of blood flow to the kidneys. Successful ischemia or reperfusion was judged by observing the change in kidney color from bright red to dark blue or from dark blue to bright red, respectively. At the end of the reperfusion period, blood samples were collected by cardiac puncture, and left nephrectomy was performed. Blood was centrifuged (3000 rpm for 10 min at 4 °C) immediately to separate the serum, which was stored at −80 °C until assayed. We fixed kidneys in 4% paraformaldehyde or immediately frozen, and stored at −80 °C for different determinations.
Assessment of renal function
An automated analyzer (Siemens ADVIA 2400, Munich, Germany) was used to measure the levels of serum creatinine (Scr) and blood urea nitrogen (BUN).
Histopathological examination
For light microscopic investigations, we fixed renal tissue specimens in 4% formalin for 24 h and embedded in paraffin. Thin sections (4 μm) were cut, deparaffinized, dehydrated and stained them with hematoxylin–eosin. We examined 10 randomly selected fields of sections from each kidney at × 400 magnification under a light microscope (Olympus, Tokyo, Japan) in a blinded manner by pathologist who was unaware of the treatment. A grading scale of 0–4, as outlined by Jablonski and coworkers,Citation21 was used for the histopathological evaluation of I/R-induced injury of the proximal tubules.
Measurement of SOD, CAT and MDA levels in kidneys
Superoxide dismutase (SOD), catalase (CAT) and malondialdehyde (MDA) levels in kidneys at 24 h after reperfusion were measured. Briefly, the kidney tissue samples were homogenized and centrifuged them at 2500 rpm for 10 min, then collected the supernatants and tested them using commercialized assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Based on the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine–xanthine oxidase system as a superoxide generator, we measured total SOD activity by the xanthine oxidase method.Citation21 We determined CAT activity by employing hydrogen peroxide to generate water and oxygen.Citation22 The results are expressed in units per milligram protein. The MDA concentrations were assayed in the form of thiobarbituric acid-reacting substances.Citation23 The results are expressed as nanomoles per milligram protein. The protein content of tissue homogenate samples was determined by BCA Protein Assay Kit (CoWin Bioscience, Beijing, China).
Measurement of myeloperoxidase (MPO) activity
MPO activity in the kidneys was measured with the commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Assessment of apoptosis
We determined the degree of apoptosis in kidneys at 24 h after reperfusion using the in situ terminal deoxynucleotidyl transferase mediated-dUTP nick end-labeling (TUNEL) assay with Cell Death Detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturers’ instructions. Briefly, paraffin sections of 4 μm thickness from tissues were de-paraffinized in xylene and hydrated in graded ethanol, permeabilized them with proteinase K (20 μg/mL) and incubated with 3% hydrogen peroxide. Next, the sections with a mixture of nucleotides and terminal transferase incubated for 1 h, and then with the converter conjugated with horseradish peroxidase for 30 min at 37 °C. The signals were measured using the 3,3′-DAB and the nuclei were counterstained with hematoxylin. The sections which deal with DNAse I or reaction without terminal deoxynucleotidyl transferase (TDT) enzyme serves as positive or negative control, respectively. Cell nuclei were stained brown color that was regarded as TUNEL-positive. The number of TUNEL-positive nuclei were counted in 10 random fields (magnification × 400) for each renal region in a blinder manner.
Immunohistochemistry analysis
Immunohistochemical staining was performed on formalin-fixed paraffin sections using the streptavidin–biotin–peroxidase method.Citation24 Briefly, 4 -μm paraffin sections of the fixed kidneys were de-parafinized in xylene and hydrated in graded ethanol, heated them in citrate buffer (0.01 mol/L, pH 6.0), and then incubated in 3% hydrogen peroxide for 15 min to block endogenous peroxidase, followed by washing in phosphate-buffered saline (PBS). The sections were then incubated with primary antibodies (caspase-3 from Santa Cruz Biotechnology, CA) at 4 °C overnight. Next, they were incubated with the secondary antibody for 30 min at room temperature, color was visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB), and the slides finally were counterstained with hematoxylin. Sections incubated without the primary antibodies were used as negative controls.
Western blotting analysis
To analyze protein expression, frozen renal tissue were homogenized and centrifuged at 14,000 rpm for 15 min at 4 °C. Equivalent amounts of protein (50 μg/Lane) were separated in a 10% SDS-PAGE gels and then electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% nonfat milk solution for 2 h at room temperature with gently shaking, and then incubated overnight at 4 °C with primary antibodies. The primary antibodies included anti-cleaved caspase-3, anti-Bcl-2 and anti-Bax, all from Santa Cruz Biotechnology (Santa Cruz, CA). Subsequently, the membranes were washed and incubated for 2 h at room temperature with a secondary antibody. The protein bands were detected by chemiluminescence technology.
Statistical analysis
All the data are presented as mean ± standard error of the mean and statistical evaluation of the data was performed by one-way analysis of variance (ANOVA). A value of p < 0.05 was considered to indicate a statistically significant difference. For statistical analysis, GraphPad Prism™ 5.0 was used (GraphPad Software; San Diego, CA).
Results
Effects of nesfatin-1 on ischemia–reperfusion-induced alterations in renal function
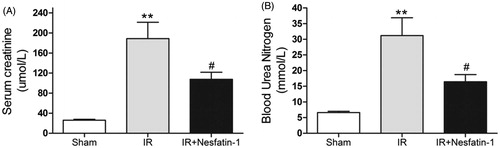
SCr and BUN levels at 24 h after reperfusion in the saline-treated I/R group were significantly increased as compared to sham control group (p < 0.01). However, nesfatin-1 administration decreased the levels of SCr and BUN at 24 h after reperfusion (p < 0.05) ().
Figure 1. Effects of nesfatin-1 on renal function at 24 h after renal I/R injury. Serum creatinine (A) and blood urea nitrogen (B) concentrations were significantly higher in the saline-treated I/R group than sham group. Pretreatment with nesfatin-1 inhibited renal dysfunction after renal I/R injury. **p < 0.01 versus the sham group and #p < 0.05 versus the I/R group.
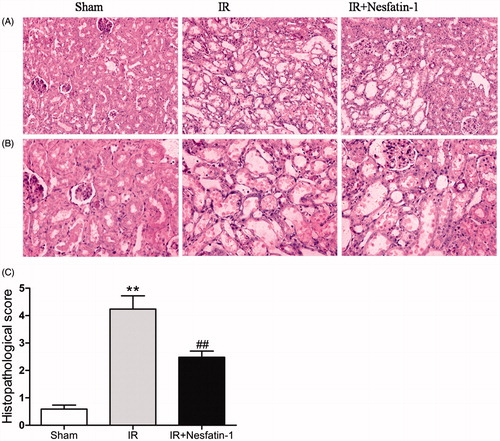
Effects of nesfatin-1 on histologic alterations caused by renal I/R
To confirm that nesfatin-1 is indeed required for renoprotective effect, we detected histologic changes at 24 h after reperfusion. The sham-operated group did not show any morphological changes. However, renal I/R group showed significantly renal alteration in renal histology, including loss of brush border, degeneration tubular structure, tubular cell swelling, tubular dilation and luminal congestion. In contrast, renal sections that were treatment of nesfatin-1 before ischemia markedly attenuated histologic damage at 24 h after reperfusion (). The histopathological score of all the three groups is presented in . The scores of the kidneys in nesfatin-1 treatment were lower than those in I/R group (p < 0.01).
Figure 2. Effects of nesfatin-1 on histology 24 h after renal I/R injury. (A) Representative photomicrographs of hematoxylin and eosin-stained kidney sections (magnification × 200). (B) magnification × 400. (C) Histopathologic score measured at 24 h after reperfusion. **p < 0.01 versus the sham group and ##p < 0.01 versus the I/R group.
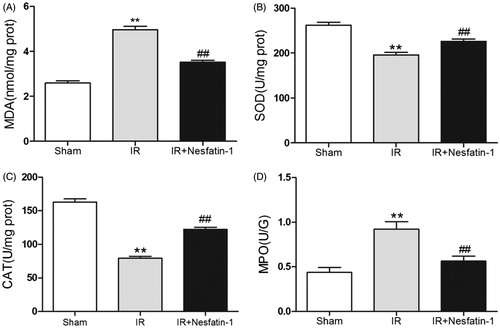
Nesfatin-1 treatment decreased oxidative stress induced by I/R injury
Reoxygenation following ischemia causes tissue oxidative stress is considered as a vital source of I/R injury, we therefore evaluated the kidney levels of MDA and antioxidant enzyme (SOD, CAT) activity. The I/R group significantly increased MDA levels (p < 0.01) and antioxidant enzyme (SOD, CAT) activity decreased (p < 0.01) at 24 h after reperfusion compared with the sham group, suggesting increases in oxidative stress. However, nesfatin-1 treatment significantly decreased the I/R-induced elevation of MDA levels (p < 0.01) and markedly ameliorated the reduction of SOD and CAT activity (p < 0.01) at 24 h after reperfusion compared with the I/R group ().
Nesfatin-1 treatment inhibits leukocytes accumulation
At 24 h of reperfusion after renal I/R injury, MPO activities in the I/R group were higher than in sham-operated group (p < 0.01), indicating that neutrophils infiltration into kidney tissue in I/R group was more apparent, while there was a dramatic reduction in MPO activities in nesfatin-1-treated group compared with I/R group (p < 0.01) ().
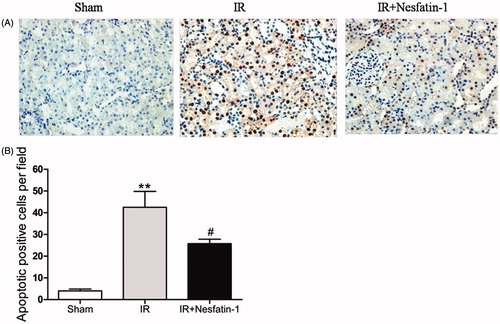
Nesfatin-1 treatment attenuated apoptosis
Since cell apoptosis is an important issue in renal I/R tubular injury,Citation25 we assessed the degree of apoptotic cells using the in situ DNA nick end labeling (TUNEL) assay and found that the TUNEL-positive cells from the I/R group were significantly increased (p < 0.01). However, compared with the I/R group, the number of TUNEL-positive cells in nesfatin-1-treated group was markedly decreased, indicating nesfatin-1 treatment significant reduction in apoptosis cells (p < 0.05) ().
Figure 4. Effects of nesfatin-1 on I/R-induced cell apoptosis at 24 h after reperfusion by TUNEL staining. (A) Representative photomicrographs of TUNEL assay (magnification × 400). (B) Quantitative analyses of apoptotic positive cells per field. **p < 0.01 versus the sham group and #p < 0.05 versus the I/R group.
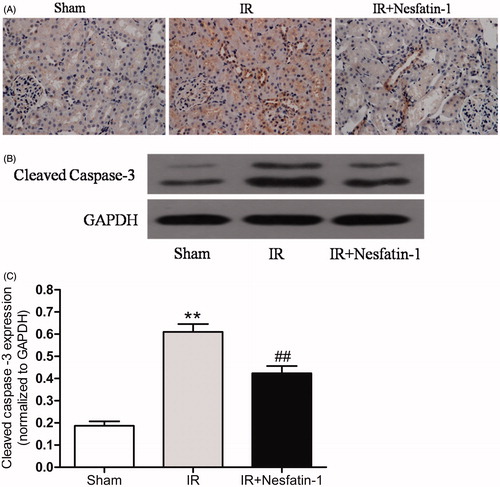
Nesfatin-1 treatment down-regulate caspase-3 activation induced by I/R injury
Immunohistochemical staining for cleaved caspase 3 in renal sections from the I/R group at 24 h after reperfusion were significantly increased, while the number of stained cells in nesfatin-1-treated group was dramatically decreased compared with the I/R group (). We further demonstrated these results by western blot analysis. The cleaved caspase-3 expression in the I/R group were markedly higher than in the sham group at 24 h after reperfusion (p < 0.01). However, nesfatin-1 treatment significantly decreased the expression of cleaved caspase-3 (p < 0.01) ().
Figure 5. Effects of nesfatin-1 on I/R-induced caspase-3 expression at 24 h after reperfusion. (A) Representative photomicrographs of immunohistochemical-stained kidney sections for cleaved caspase-3 (magnification × 400). (B) Representative western blots of cleaved caspased-3. (C) Quantitative analyses of the band density of cleaved caspased-3 (relative to GAPDH). **p < 0.01 versus the sham group and ##p < 0.01 versus the I/R group.
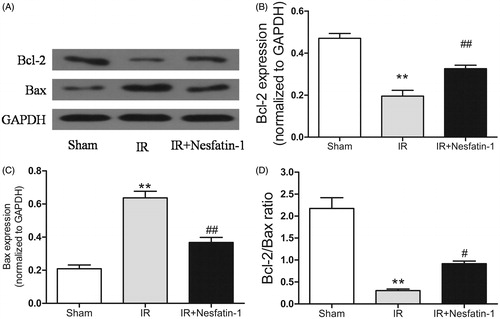
Nesfatin-1 treatment increases the Bcl-2/Bax ratio following I/R injury
We used western blot analysis to evaluate the Bcl-2 and Bax protein expression, which reflect mitochondrial stress, indicated that nesfatin-1 downregulated the levels of the pro-apoptotic proteins Bax, while upregulating the levels of the anti-apoptotic protein Bcl-2 (). Our results showed that the Bcl-2/Bax ratio was markedly decreased in I/R group compared with sham-operated group (p < 0.01) and the ratio was dramatically increased in nesfatin-1-treated group (p < 0.05) ().
Figure 6. Effects of nesfatin-1 on Bcl-2 and Bax expression, and Bcl-2/Bax ratio at 24 h after reperfusion. (A) Representative western blots of Bcl-2 and Bax expression. (B and C) Quantitative analyses of the band density of Bcl-2 and Bax. (D) Alterations in the Bcl-2/Bax ratio. **p < 0.01 versus the sham group and #p < 0.05 versus the I/R group and ##p < 0.01 versus the I/R group.
Discussion
To our knowledge, this study is the first to demonstrate the protective effects of nesfatin-1 on acute renal injury in a rat model, and its protective effects was largely due to the inhibition of the oxidative stress and tubular apoptosis.
Renal ischemia followed by reperfusion still remains a serious problem in clinical procedures, such as hemorrhagic shock, organ procurement, hydronephrosis, vascular surgery, accidental or iatrogenic trauma, sepsis, partial nephrectomy and renal transplantation. Furthermore, renal I/R injury is an important cause of acute kidney injury (AKI). It contributes to the development of acute renal failure (ARF), which is associated with high mortality and morbidity in patients with transplanted kidney.Citation3 A substantial amount of study to date has focused on the mechanism of oxidative stress and cell apoptosis in renal I/R injury, so many therapeutic method aimed at oxidative stress and apoptosis has been reported to protection renal injury.Citation26–28 Nesfatin-1, an 82-amino-acid peptide, has been reported to possess antioxidative, anti-inflammation and anti-apoptotic properties. Previous study showed the neuroprotection of nesfatin-1 against SAH-induced injury in rats by inhibiting inflammation and apoptosis.Citation18 Furthermore, Kolgazi et al. reported that nesfatin-1 alleviates gastric damage via antioxidant mechanisms.Citation17 So consistent with these reports, in the present study we first demonstrated that nesfatin-1 pretreatment significantly ameliorated serum SCr, BUN and histopathologic damage by attenuate oxidative stress and apoptotic cell death.
Previous study has been demonstrated that ROS plays a critical role in the pathological process of renal IR injury.Citation29 ROS can react with proteins, lipids, nucleic acids, trigger inflammation, as well as induced tubular cell apoptosis.Citation30–32 Thus, various exogenous antioxidant agents have received abundant attention and applied to reduce renal IR injury. As a peptide, nesfatin-1 was shown to inhibit oxidative stress,Citation17 suggesting that nesfatin-1 might ameliorate renal I/R injury. We hypothesized that nesfatin-1 would protect kidney from I/R injury by reducing oxidative stress. Our results showed that nesfatin-1 treatment causes a significant inhibition MDA production, which is a good indicator of oxidative stress-mediated lipid peroxidation, while the MDA levels are dramatically increased in I/R group. SOD and CAT are the most important endogenous antioxidant enzymes, which can act against the harmful effects of free oxygen radicals,Citation5 so we examined these two enzymes to evaluate the enzymatic antioxidant status of I/R-induced injury in rat kidneys. Our results showed that decreased SOD and CAT values were improved by pretreatment with nesfatin-1. As a result, the enhanced SOD and CAT activity induced by nesfatin-1 may help to attenuating kidney MDA levels after renal I/R.
Studies reported that myeloperoxidase (an enzyme located in leucocytes) is used as an indicator of accumulation of neutrophils in renal I/R.Citation33 In our experiment, the level of myeloperoxidase in kidney markedly increased after renal I/R injury. We have also found that nesfatin-1 treatment significantly reduced the values of myeloperoxidase, indicating that nesfatin-1 protected the kidneys against renal I/R injury by inhibits activated leukocytes infiltrate into renal tissue.
Renal I/R injury is a dynamic process that involves several cell types and molecular mediators. In renal I/R injury, the anti-apoptotic protein declined and oxidative damage to the enzyme complexes triggered apoptosis. Studies have demonstrated that renal tubular cell apoptosis plays an important role in renal I/R injury and contributes to renal dysfunction.Citation25,Citation34,Citation35 In addition, abrogation of apoptosis ameliorates post-reperfusion injury and improved renal function and survival.Citation25,Citation36 The present study showed that the kidney of the I/R group has more TUNEL-positive cells than the sham group. Nesfatin-1 treatment dramatically alleviated TUNEL-positive cells in the I/R rats. Decreased apoptosis is associated with the deactivation of a caspase cascade. Previous study reported that caspase-3, which is regarded as a pivotal indicator of apoptosis, had an important role in the process of apoptosis in renal I/R injury models.Citation37,Citation38 Nesfatin-1 has been reported to inhibit active caspase-3 expression in vivo.Citation18 Consistent with these reports, we found that nesfatin-1 significantly inhibited the activation of caspase-3. The mitochondrion is the central organelle in the intrinsic apoptosis pathway.Citation39 Mitochondrial injury activates mitochondrial-mediated apoptosis and down-regulates the Bcl-2/Bax ratio. Decreasing the Bcl-2/Bax ratio will change mitochondrial membrane permeability, and promotes the release of cytochrome c and caspase-3 mediated renal tubular apoptosis.Citation40,Citation41 We found that nesfatin-1 down-regulated Bax and up-regulated Bcl-2 expression, indicating nesfatin-1 treatment increased I/R-induced reduction in the Bcl-2/Bax ratio.
There are several limitations to our study worth noting. First, the sample size of this study was small, large sample size is needed in further studies. Second, we examined all the parameters taken only 24 h after reperfusion in this model, without evaluating the long-term roles of nesfatin-1. Third, this study used an animal model of I/R injury, thus the outcomes may be different from those in humans. Additional studies are necessary to address these issues.
In summary, this study is the first to demonstrate that pretreatment with nesfatin-1 protects rat from renal I/R injury through antioxidant and anti-apoptosis properties. Our findings suggest that nesfatin-1 may provide a novel therapeutic potential for the improvement of renal I/R injury.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Grace PA. Ischemia–reperfusion injury. Br J Surg. 1994;81:637–647
- Anaya-Prado R, Toledo-Pereyra LH, Lentsch AB, Ward PA. Ischemia/reperfusion injury. J Surg Res. 2002;105:248–258
- Dorai T, Fishman AI, Ding C, Batinic-Haberle I, Goldfarb DS, Grasso M. Amelioration of renal ischemia–reperfusion injury with a novel protective cocktail. J Urol. 2011;186:2448–2454
- Weight SC, Bell PR, Nicholson ML. Renal ischemia–reperfusion injury. Br J Surg. 1996;83:162–170
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164
- Meldrum DR, Ayala A, Wang P, Ertel W, Chaudry IH. Association between decreased splenic ATP levels and immunodepression: Amelioration with ATP-MgCl2. Am J Physiol. 1991;261:R351–R357
- Rabb H, O'Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468
- Wang P, Ba ZF, Meldrum DR, Chaudry IH. Diltiazem restores cardiac output and improves renal function after hemorrhagic shock and crystalloid resuscitation. Am J Physiol. 1992;262:H1435–H1440
- Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation. 1989;80:1115–1127
- Oh IS, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712
- Cowley MA, Grove KL. To be or NUCB2, is nesfatin the answer? Cell Metab. 2006;4:421–422
- Colmers WF. Less fat with nesfatin. Trends Endocrinol Metab. 2007;18:131–132
- Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology (Berl). 2008;201:115–123
- Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD. Central nesfatin-1-expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflamm. 2009;6:27–35
- Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood–brain barrier without saturation. Peptides. 2007;28:2223–2228
- Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood–brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372–2381
- Kolgazi M, Cantali-Ozturk C, Deniz R, et al. Nesfatin-1 alleviates gastric damage via direct antioxidant mechanisms. J Surg Res. 2015;193:111–118
- Ozsavci D, Ersahin M, Sener A, et al. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage-induced oxidative brain damage in rats. Neurosurgery. 2011;68:1699–1708; discussion 1708
- Angelone T, Filice E, Pasqua T, et al. Nesfatin-1 as a novel cardiac peptide: Identification, functional characterization, and protection against ischemia/reperfusion injury. Cell Mol Life Sci. 2013;70:495–509
- Liu X, Chen H, Zhan B, et al. Attenuation of reperfusion injury by renal ischemic postconditioning: The role of NO. Biochem Biophys Res Commun. 2007;359:628–634
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500
- Ji HJ, Hu JF, Wang YH, Chen XY, Zhou R, Chen NH. Osthole improves chronic cerebral hypoperfusion induced cognitive deficits and neuronal damage in hippocampus. Eur J Pharmacol. 2010;636:96–101
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358
- Chatterjee PK, Zacharowski K, Cuzzocrea S, et al. Lipoteichoic acid from Staphylococcus aureus reduces renal ischemia/reperfusion injury. Kidney Int. 2002;62:1249–1263
- Bonegio R, Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 2002;11:301–308
- Chen H, Xing B, Liu X, et al. Ischemic postconditioning inhibits apoptosis after renal ischemia/reperfusion injury in rat. Transplant Int. 2008;21:364–371
- Qiao X, Li RS, Li H, et al. Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress. Am J Physiol Renal Physiol. 2013;304:F112–F119
- Koc M, Kumral ZN, Ozkan N, et al. Obestatin improves ischemia/reperfusion-induced renal injury in rats via its antioxidant and anti-apoptotic effects: Role of the nitric oxide. Peptides. 2014;60C:23–31
- Aragno M, Cutrin JC, Mastrocola R, et al. Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: Attenuation by dehydroepiandrosterone. Kidney Int. 2003;64:836–843
- Singh D, Chopra K. The effect of naringin, a bioflavonoid on ischemia–reperfusion induced renal injury in rats. Pharmacol Res. 2004;50:187–193
- Nakagawa K, Koo DD, Davies DR, et al. Lecithinized superoxide dismutase reduces cold ischemia-induced chronic allograft dysfunction. Kidney Int. 2002;61:1160–1169
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210
- Chatterjee PK, Todorovic Z, Sivarajah A, et al. Inhibitors of calpain activation (PD150606 and E-64) and renal ischemia–reperfusion injury. Biochem Pharmacol. 2005;69:1121–1131
- Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol. 2003;23:511–521
- Yamamoto K, Tomita N, Yoshimura S, et al. Hypoxia-induced renal epithelial cell death through caspase-dependent pathway: Role of Bcl-2, Bcl-xL and Bax in tubular injury. Int J Mol Med. 2004;14:633–640
- Daemen MA, van ‘t Veer C, Denecker G, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549
- Yang B Jain, S, Ashra SY, Furness PN, Nicholson ML. Apoptosis and caspase-3 in long-term renal ischemia/reperfusion injury in rats and divergent effects of immunosuppressants. Transplantation. 2006;81:1442–1450
- Yang C, Jia Y, Zhao T, et al. Naked caspase 3 small interfering RNA is effective in cold preservation but not in autotransplantation of porcine kidneys. J Surg Res. 2013;181:342–354
- Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136
- Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285