Abstract
Aim: Endoplasmic reticulum (ER) stress and unfolded protein response (UPR) are implicated in many fibrotic diseases, including renal fibrosis. Whether Ginsenoside-Rg1 (G-Rg1) could attenuate renal fibrosis via suppression of ER stress and UPR has not been reported. The aim of this study was to explore the effect of G-Rg1 on ER stress and UPR-induced apoptosis in kidneys with unilateral ureteral obstruction (UUO) rat model. Methods: Twenty-four male Sprague–Dawley rats were randomly divided into control group, model group and G-Rg1 treatment group. G-Rg1 was administered to rats by intraperitoneal injection. Renal interstitial fibrosis in the model group was developed by UUO in rats. Renal function was estimated by the levels of serum creatinine (Scr) and blood urea nitrogen (BUN). Renal pathological damage was evaluated by hematoxylin and eosin (HE) and Masson’s trichrome staining. The ER stress was assessed with glucose-regulated protein (GRP) 78 expression, and the proapoptotic response was detected with CCAAT/enhancer-binding protein homologous protein (CHOP) and caspase-12 expressions by Western Blot. The number of apoptotic cells was determined by Terminal-deoxynucleotidyl Transferase Mediated Nick End Labeling (TUNEL) analysis. Results: UUO for 14 days aggravated renal function, renal damage and renal interstitial fibrosis, activated ER stress response (induction of GRP78 protein), enhanced the proapoptotic response (increase in CHOP and caspase-12 proteins) and increased the number of apoptotic cells (shown by the TUNEL assay). Treatment with G-Rg1 significantly ameliorates the renal pathological lesions and decreases expressions of ER stress-associated proteins and the level of apoptotic cells in kidneys. Conclusion: G-Rg1 suppresses renal cell apoptotic and fibrotic process partly through inhibition of ERS- and UPR-related apoptotic pathway in the kidneys after UUO.
Introduction
Renal interstitial fibrosis (RIF), the main common consequence of chronic kidney disease (CKD) progressing to end-stage renal disease,Citation1 is a multistage and complicated process driven by various cytokines and substances, and the exact mechanism is still not be fully understood.Citation2 Only a few anti-fibrotic agents are available to inhibit RIF in clinical practice.Citation3 The inhibition and reversal of RIF depend on the further study concerning the mechanism implicated in the development of RIF. Endoplasmic reticulum (ER) stress is an adaptive response to handle the accumulated unfolded proteins in ER lumen under abnormal conditions, such as hypoxia, ischemia and oxidative stress.Citation4 If the stressors are not removed or prolonged, apoptotic pathway is activated and cell apoptosis subsequently occurs.Citation5 Emerging data show persistent ER stress is involved in the pathogenesis of CKD and lead to renal tubular cell apoptosis and eventually renal interstitial fibrosis.Citation6 It maybe the new mechanism that contributes to the progression of RIF.Citation7,Citation8
Ginsenoside-Rg1 (G-Rg1) is believed to be the main active component of Panax ginseng, a traditional Chinese herb, which poses antioxidant, anti-proliferative and anti-apoptotic activities and is used extensively to treat neurological, cardiovascular, hepatic and inflammation or immune related diseases.Citation9,Citation10 Related studies demonstrate that G-Rg1 can markedly inhibit organ fibrosis, including myocardial fibrosisCitation11 and liver fibrosis.Citation12 The anti-fibrotic activity of G-Rg1 on renal fibrosis has been studied as well. Recent evidences manifest sustained ER stress-induced apoptosis play a significant role in the development of renal fibrosis. However, the role of G-Rg1 on ER stress has not been reported. Therefore, this study was to illustrate the effects of G-Rg1 on ER stress markers and renal fibrosis in UUO rat model.
Materials and methods
Experimental reagents
G-Rg1 was produced by Beijing Institute of Natural Medicine. Rabbit anti-glucose-regulated protein (GRP) 78 antibody were purchased from Abcam (Hong Kong, China). Rabbit anti-caspase-12 antibody was purchased from Sigma-Aldrich (St. Louis, MO). Mouse anti-CHOP mAb was purchased from cell signaling Technology (Boston, MA). Polyclonal goat anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from GenScript (Piscataway, NJ). Horseradish peroxidase (HRP)-conjugated second antibody anti-rabbit IgG and anti-goat IgG was purchased from Solarbio (Beijing, China). HRP-conjugated anti-mouse IgG was purchased from ZSGB Bio (Beijing, China).
Animals and development of UUO rat model
Twenty-four Sprague–Dawley male rats, with body weight 220 ± 30 g, were purchased from Shanghai Laboratory Animal Research Center. All rats were divided equally into control group (control), model group (model) and G-Rg1 treatment group (G-Rg1), each consisting of eight rats. Renal interstitial fibrosis of rats was induced by UUO surgery.Citation13 Anesthetized animals underwent the flank incision and the left kidney and ureter were isolated. The left ureter was ligated at two points and then cut between the two ligated points. Rats in control group were performed in the identical way but the left ureter was not ligated or cut. G-Rg1 was given to animals in G-Rg1 treatment group by intraperitoneal injection and dose of G-Rg1 was 50 mg/kg per day according to the literature.Citation14 The same volume of physical saline was administered to other rats in the same way. Rats were killed at d 14 after UUO surgery. Part of the obstructed kidney tissues was placed in 10% paraformaldehyde and then paraffin-embedded for hematoxylin–eosin (HE) and Masson’s trichrome staining, and the remaining tissue was stored in liquid nitrogen for later protein exertion to analyze the different activation of ER stress proteins.
The measurement of Scr and BUN
Blood samples were collected and centrifuged at 6000g for 5 min. The levels of serum creatinine (Scr) and blood urea nitrogen (BUN) were determined by automatic biochemistry analyzer (Glamour 2000, America).
Renal pathological lesions and semiquantitative analysis of renal fibrosis
Renal tissues fixed with 10% formalin were dehydrated in alcohol and embedded in paraffin. The processed tissues were cut into slices (4 μm). HE and Masson's trichrome staining were performed according to the protocol. Renal injury index including interstitial fibrosis, inflammatory, cell infiltration, interstitial edema, tubular atrophy, cell vacuolar degeneration and tubular expansion were measured to assess the renal interstitial lesions. Each parameter was evaluated and given a score from 0 to 4+ (0, no changes; 1+, changes affecting <25% of the sample; 2+, changes affecting 25–50%; 3+, changes affecting 50–75%; 4+, changes affecting 75–100%).Citation15 Ten different fields were selected and scanned to estimate the level of renal interstitial fibrosis with HE staining using Image Pro plus-6.0 software (Silver Spring, MD).Citation16 The percentage of blue collagen area with Masson’s trichrome staining in the renal tissue was measured as well.
The detection of kidney GRP78, CHOP and caspase-12
Protein lysates were separated by 12% SDS gel electrophoresis, and then transferred onto PVDF membranes. After blocking with 5% milk overnight at 4°C, membranes were incubated with corresponding primary antibodies (GRP78, CHOP and caspase-12 at concentrations of 1:1000) for 2 h at room temperature, respectively. Horseradish peroxidase (HRP)-conjugated secondary antibodies (1:200 dilution) were incubated afterward for 1 h, and detection of band densities was by enhanced chemiluminescence (Beyotime, Nantong, China), and then analyzed by Quantity One software (Bio-Rad, Hercules, CA). For quantification, relative optical densities of GRP78, CHOP and caspase-12 protein bands in each lane were normalized to those of GAPDH.
The determination of apoptotic cells
Apoptosis of tubular and interstitial cell was detected by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay by using FragEL™ DNA Fragmentation Detection Kit (Merck, Darmstadt, Germany) in accordance with the manufacturer's protocols. The sections were observed under an Olympus immunofluorescence microscope under × 400 magnification. The number of apoptotic cells was counted by selecting more than 10 fields.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Statistical comparisons from each group were assessed by one-way analysis of variance (ANOVA), followed by the least-significant-difference (LSD) test as a post-hoc test. A value of p < 0.05 was considered to be significant. All data were performed using the SPSS 19.0 software.
Results
The levels of Scr and BUN
BUN and Scr levels in UUO group were significantly higher than those in the control group (BUN: 70.68 ± 6.23 mmol/L vs. 29.61 ± 2.42 mmol/L, p < 0.05; Scr: 12.89 ± 2.70 μmol/L vs. 5.83 ± 0.51 μmol/L, p < 0.05). G-Rg1 administration decreased BUN and Scr levels (44.86 ± 5.71 mmol/L vs. 71.38 ± 6.44 mmol/L, p < 0.05; 7.41 ± 1.01 μmol/L vs. 12.89 ± 2.70 μmol/L, p < 0.05).
Renal pathological changes
The kidneys of rats in control group showed normal histology. UUO rats exhibited interstitial fibrosis, cellular infiltration, tubular ectasia, atrophy, necrosis and epithelial cell degeneration (). Pathological changes were evaluated by renal tubular injury index. As shown in , UUO enhanced the renal tubular injury index about six times but this elevation was remarkably attenuated (0.56 times that of model group) by the administration of G-Rg1 ().
Figure 1. G-Rg1 minimized UUO-triggered pathological changes in the kidneys. (A) Representative photomicrographs stained by HE (upper panels) and Masson’s trichrome (lower panels) (original magnification 200×). (B) The semi-quantitative accession of the renal interstitial lesions in HE-stained sections. (C) The degree of interstitial collagen deposits in Masson’s trichrome-stained sections. *p < 0.01 versus control group; #p < 0.01 versus model group.
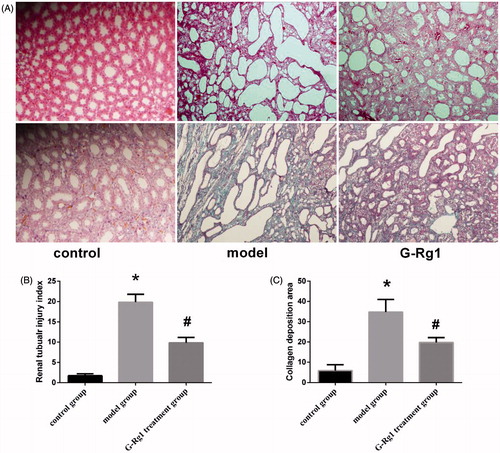
Masson's trichrome staining showed that few collagens were deposited in the normal kidney, mainly located in the glomerular basement membrane, mesangial region and around renal tubular capillaries. In the model group rats, amount of collagen deposition was observed, and the collagen was widely distributed in the mesenchymal area in interstitium. G-Rg1 treatment remarkably blunted the interstitial collagen deposition (). In terms of the degree of blue collagen deposition, only small amounts of collagen deposition were detected in control group samples, whereas UUO increased about 11-fold. Interestingly, the administration of G-Rg1 weakened its degree (). Taken together, these results support the assertion that G-Rg1 treatment can markedly ameliorate renal pathological lesions and reduce the amount of collagen accumulation in UUO rats.
The states of apoptotic cells in kidney
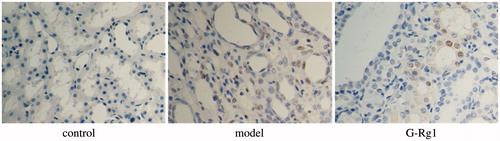
TUNEL-positive cells in the cortex increased from 0.5/high power field (HPF) to 31/HPF after UUO surgery, whereas the administration of G-Rg1 decreased the TUNEL-positive cells to 13/HPF ().
Expressions of GRP78 in rat kidneys among groups
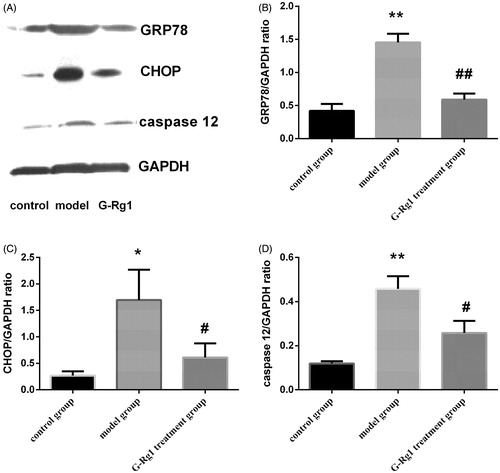
As shown in , GRP78, a representation chaperon of ER stress, was induced remarkably after UUO (1.45 ± 0.07 vs. 0.41 ± 0.06, p < 0.01). Expression of GRP78 in the G-Rg1 treatment group was lower than that of in the model group (0.59 ± 0.05 vs. 1.45 ± 0.07, p < 0.05).
Figure 3. Effect of G-Rg1 on the expression of GRP78, CHOP and caspase 12 proteins in renal tissue. Expressions of GRP78 (78 kD), CHOP (27 kD), caspase 12 (42 kD) and GAPDH (36 kD) were determined by western blot. Respective images are shown in (A) and quantification of relative densitometries is shown in (B) (GRP78), (C) (CHOP), (D) (caspase 12), respectively. *p < 0.05, **p < 0.01 versus control group; #p < 0.05, ##p < 0.01 versus model group.
Expressions of CHOP and caspase-12 in rat kidneys among groups
shows the expression of CHOP protein in experimental groups. CHOP protein was significantly increased in the model group compared with the control group (1.69 ± 0.33 vs. 0.27 ± 0.05, p < 0.05) but decreased in G-Rg1 treatment group (0.61 ± 0.15 vs. 1.69 ± 0.33, p < 0.05). Similar results were observed for caspase-12 expression among groups (). Weak expression of caspase-12 was observed in control group. UUO induced a significant increase in caspase-12 expression (0.46 ± 0.33 vs. 0.12 ± 0.01, p < 0.01) while G-Rg1 down-regulated its expression (0.26 ± 0.31 vs. 0.46 ± 0.33, p < 0.05).
Discussion
In eukaryotic cells, ER prompts the unfolded proteins in ER lumen to proper folding and transferred to Golgi complex for further processing.Citation4 When ER function is disrupted by increased accumulated unfolded proteins due to hypoxia, ischemia and oxidant stress, the unfolded proteins release molecular chaperones from the membrane such as GRP78, and lead to ER stress and UPR. The activation of ER stress and UPR enhances capacity of protein folding, reduces accumulation and accelerates the degradation of the unfolded protein.Citation17 Concisely, ER stress and UPR serve as a protective mechanism to restore the ER homeostasis. In contrast, persistent ER stress triggered the proapoptotic UPR, mainly mediated by CHOP- and caspase-12-dependent apoptotic pathway.Citation18 CHOP, also known as growth arrest and DNA damage inducible genes (GADD153), is an important intermediate protein linking ER stress to apoptosis.Citation19 Caspase-12, which is exclusively located in the ER membrane,Citation20 is absolutely activated by ER stress-induced factors, not other death stimuli.Citation21
Previous studies have shown that ER stress-related apoptosis may be the potential mechanism for tubular epithelial cell atrophy and renal fibrosis.Citation22,Citation23 Furthermore, recent evidence revealed that ER stress and UPR have been closely related to fibrotic process and at least, partly regarded as a potential pro-fibrotic stimulus.Citation24,Citation25 UUO can induce renal interstitial fibrosis rapidly and mostly used to study the mechanism of renal fibrosis. Recent researches have demonstrated prolonged overwhelming ER stress and UPR-related apoptosis caused the damage to kidneys in UUO rats and involves in the progression of kidney fibrosis in UUO rats.Citation26 Targeting ER stress and UPR-related apoptosis may be the novel mechanism that retards renal interstitial fibrosis.
G-Rg1, purified from P. ginseng, has been reported to effectively inhibit organ fibrosis.Citation11,Citation12 In addition, Xie et al. elaborated that G-Rg1 ameliorated renal interstitial fibrosis by inhibiting the tubular epithelial-myofibroblast transitionCitation27 and decreasing the expression of thrombospondin-1.Citation14 Herein, we explored the role of G-Rg1 therapy on ER stress-induced apoptosis and renal interstitial injury in a rat model of UUO. Our results showed UUO led to the deterioration of renal function by decreasing excretion of Scr and BUN. HE staining results showed tubular dilation, epithelial cell degeneration and necrosis, interstitial fibrosis and inflammatory cell infiltration were exacerbated at d 14 after UUO (). On the other hand, UUO caused mass collagen deposition and wide-spread interstitial fibrosis as shown by Masson's trichrome staining (). These pathological changes were significantly attenuated and renal function was improved by the administration of G-Rg1. To discover the effect of G-Rg1 on ER-associated apoptotic signals in UUO rat kidneys, we assessed the expression of GRP78 and its downstream proapoptotic factors including CHOP and caspase-12. In addition, TUNEL analysis was performed to evaluate the level of apoptotic cells. As shown in , GRP78 protein, accompanied by the expressions of proapoptotic proteins CHOP and caspase-12, was activated after UUO. Meanwhile, the number of apoptotic cells showed by TUNEL has increased significantly. Interestingly, G-Rg1 administration inhibited GRP78 expression and followed by diminished CHOP and caspase-12. Besides, G-Rg1 treatment reduced the number of apoptotic cells observed by TUNEL staining. Therefore, G-Rg1 suppresses cell apoptotic process and interstitial fibrosis possibly by inhibiting ER stress induced apoptotic pathway in the kidney after UUO. This is a potential mechanism that G-Rg1 inhibits renal interstitial fibrosis. However, we do not explore the other apoptotic pathways such as death receptor or mitochondria pathways in this study, so other apoptotic pathways that are not investigated cannot be excluded.
In conclusion, UUO-stimulated ER stress and enhance UPR-induced apoptosis. G-Rg1 therapy ameliorates renal cell apoptosis and fibrotic process, possibly via inhibition of overwhelming ER stress pathway after UUO. More researches are still needed to illustrate the exact effect of G-Rg1 on ER stress, apoptosis and renal fibrosis.
Declaration of interest
This work was supported by the Social Development Foundation of Kunshan (ks1228) and the Clinical and Technology Development Fund of Jiangsu University (JLY20120046).
References
- Farris AB, Colvin RB. Renal interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol Hypertens. 2012;21:289–300
- Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834
- Boor P, Ostendorf T, Floege J. Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–656
- Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529
- Chiang CK, Hsu SP, Wu CT, et al. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol Med. 2011;17:1295–1305
- Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta. 2013;1832:940–947
- Inagi R, Ishimoto Y, Nangaku M. Proteostasis in endoplasmic reticulum – New mechanisms in kidney disease. Nat Rev Nephrol. 2014;10:369–378
- Hon CC, Chow YC, Zeng FY, Leung FC. Genetic authentication of ginseng and other traditional Chinese medicine. Acta Pharmacol Sin. 2003;24:841–846
- Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry. 2011;72:689–699
- Wei HJ, Yang HH, Chen CH, et al. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release. 2007;120:27–34
- Geng J, Peng W, Huang Y, Fan H, Li S. Ginsenoside-Rg1 from Panax notoginseng prevents hepatic fibrosis induced by thioacetamide in rats. Eur J Pharmacol. 2010;634:162–169
- Ma FY, Tesch GH, Ozols E, Xie M, Schneider MD, Nikolic-Paterson DJ. TGF-β1-activated kinase-1 regulates inflammation and fibrosis in the obstructed kidney. Am J Physiol Renal Physiol. 2011;300:F1410–F1421
- Xie XS, Liu HC, Wang FP, et al. Ginsenoside Rg1 modulation on thrombospondin-1 and vascular endothelial growth factor expression in early renal fibrogenesis in unilateral obstruction. Phytother Res. 2010;24:1581–1587
- Remuzzi G, Zoja C, Gagliardini E, Corna D, Abbate M, Benigni A. Combining an antiproteinuric approach with mycophenolate mofetil fully suppresses progressive nephropathy of experimental animals. J Am Soc Nephrol. 1999;10:1542–1549
- Mizuguchi Y, Miyajima A, Kosaka T, Asano T, Asano T, Hayakawa M. Atorvastatin ameliorates renal tissue damage in unilateral ureteral obstruction. J Urol. 2004;172:2456–2459
- Kitamura M. The unfolded protein response triggered by environmental factors. Semin Immunopathol. 2013;35:259–275
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: Cell life and death decisions. J Clin Invest. 2005;115:2656–2664
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885
- Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894
- Fang L, Xie D, Wu X, Cao H, Su W, Yang J. Involvement of endoplasmic reticulum stress in albuminuria induced inflammasome activation in renal proximal tubular cells. PLoS One. 2013;8:e72344
- Johnson A, DiPietro LA. Apoptosis and angiogenesis: An evolving mechanism for fibrosis. FASEB J. 2013;27:3893–3901
- Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol. 2009;112:e1–e9
- Inagi R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr Opin Pharmacol. 2010;10:156–165
- Yeh CH, Chiang HS, Lai TY, Chien CT. Unilateral ureteral obstruction evokes renal tubular apoptosis via the enhanced oxidative stress and endoplasmic reticulum stress in the rat. Neurourol Urodyn. 2011;30:472–479
- Xie XS, Yang M, Liu HC, et al. Influence of ginsenoside Rg1, a panaxatriol saponin from Panax notoginseng, on renal fibrosis in rats with unilateral ureteral obstruction. J Zhejiang Univ Sci B. 2008;9:885–894