Abstract
Background: Evidences suggest a role of renin–angiotensin system (RAS) in the development of chronic allograft injury. Methods: We correlated intrarenal angiotensin-converting enzyme, angiotensin II (Angio II) and transforming growth factor β1 (TGFβ1) expression in 58 biopsies-proven chronic allograft nephropathy (CAN) with tissue injury and allograft survival. Results: The biopsies with CAN were graded according to Banff classification as I (22 cases), II (17) and III (19); 27 biopsies also showed a mononuclear inflammatory infiltrate in scarred areas. There were increased expression of angiotensin converting-enzyme (ACE), Angio II and TGFβ1 mainly in tubulointerstitial compartment in the group with CAN; there was no association of Angio II and TGFβ1 expression with interstitial fibrosis. There were no significant differences of ACE, Angio II and TGFβ1 expression between the patients treated and untreated with RAS blockade, and with the graft outcome. Interstitial inflammatory infiltrate had positive correlation with interstitial fibrosis and significant impact on graft survival at 8 years. Conclusions: Our study showed in a group of cases with CAN a high percentage of inflammatory infiltrate that correlated with interstitial fibrosis and graft outcome. The chronic inflammatory changes in these cases did not show significant association with local RAS expression.
Introduction
Chronic allograft nephropathy (CAN) is the most prevalent cause of renal allograft loss after the first year following transplantation. The patients show a gradual and progressive deterioration of renal function, proteinuria and hypertension. Histologically, the lesion is characterized by unspecific findings, such as glomerulosclerosis, tubular atrophy, interstitial fibrosis and intimal arterial hyperplasia. The pathogenesis remains uncertain.Citation1–3 There are recent evidences suggesting a role of the renin–angiotensin system (RAS) in the development of progressive chronic allograft injury. The intrarenal RAS has been recognized as a growth promoter that contributes to renal fibrosis and arteriosclerosis with deterioration of graft function. Angiotensin II (Angio II), a locally renal produced growth factor, regulates recruitment and activation of inflammatory and resident cells with increased synthesis of extracellular matrix.Citation4,Citation5 These effects are mainly mediated by up-regulation of other growth factors, specially transforming growth factor β1 (TGFβ1).Citation6,Citation7 There are also evidences that chronic cyclosporine nephrotoxicity is mediated by RAS and TGFβ1.Citation8,Citation9 Some studies have been shown that RAS blockade retards interstitial fibrosis and disease progression with increase of graft survival in CAN.Citation10–12 There are a few studies that demonstrate gene and protein expression of RAS components in CAN cases.Citation13–16
The aim of this study was to revise our CAN cases and analyze the renal distribution of angiotensin converting-enzyme (ACE), Angio II and TGFβ1 and correlate with tissue injury and allograft survival.
Subjects and methods
Patients
From 338 patients who received a renal allograft at Botucatu Medical School University Hospital (Botucatu-SP/Brazil) in the period from December 1987 to December 2007, we selected 58 patients with a biopsy-proven CAN after 6 months of transplantation. All patients consent to participate in the study.
The following clinical data were analyzed in the medical records of the patients: gender, age at transplantation, native kidney disease, donor type, human leukocyte antigen (HLA) matching (defined as the number of HLA-matched antigens at loci A, B and DR), cold ischemia time, delayed graft function, immunosuppression therapy, number of rejection episodes and serum creatinine at 6 months and 1 year post-transplant, at biopsy and at the end of the follow-up ().
Table 1. Clinical characteristics of patients with chronic allograft nephropathy (CAN).
Chronic allograft dysfunction was defined as a gradual deterioration of graft function with serum creatinine more than 1.4 mg/dL, and/or the presence of proteinuria and delayed graft function as return to dialysis in the first week after transplantation. Graft loss was defined as the resumption of dialysis, and survival was censored for patients dying with functional grafts. The 1-year graft survival rate was analyzed for all renal allograft cases.
Initial immunosuppressive treatment included: azathioprine, prednisone and cyclosporine in 53.7% of the patients and mycophenolate mofetil and cyclosporine in 14.8%; the others received mycophenolate mofetil and prednisone (seven patients), azathioprine and prednisone (four patients), mycophenolate, prednisone and tacrolimus (five patients), azathioprine, prednisone and tacrolimus (two patients), azathioprine, prednisone and cyclosporine (one patient). In the final follow-up, 45.6% of the patients were on sodic mycophenolate and prednisone and 14% with sirolimus, prednisone and sodic mycophenolate. The others were receiving mycophenolate mofetil and prednisone (6), sirolimus and prednisone (5), mycophenolate mofetil, prednisone and cyclosporine (3), mycophenolate mofetil, prednisone and sirolimus (2), sirolimus, prednisone and tacrolimus (2), sirolimus and azathioprine (1), sirolimus and sodic mycophenolate (1), prednisone, azathioprine and cyclosporine (1) and sodic mycophenolate, prednisone and tacrolimus (1). At the time of the biopsy, 20 patients have been treated with RAS blockade. During the follow-up period of this study (113.02 ± 53.1 months), seven patients lost their graft and six patients died with functional allografts.
Histopathology
Tissue samples were fixed in Duboscq-Brazil, paraffin-embedded and then 3 µm sections were processed for routine histological staining (hematoxylin–eosin, periodic acid-Schiff, silver methenamine and Masson's trichrome). The selected graft biopsies were reviewed by one renal pathologist and evaluated according to Banff criteria.Citation17 The control group included 20 zero-time biopsies.
Immunohistochemistry
The primary antibodies used in this study recognized: (1) Markers of RAS: mouse anti-human ACE monoclonal antibody (3C5-AB11737, Abcam Inc., Abcam, MA), rabbit anti-human Angio II monoclonal antibody (T-4007, Bachem, Península Laboratories, Inc., San Carlos, CA); (2) Marker of cell growth with extracellular matrix deposition: mouse anti-human TGFβ1 monoclonal antibody (TB21-AB1279, Abcam, Inc., Abcam, MA).
For immunohistochemical studies, 3 µm tissue sections were mounted on silanated slides, dried for 15 hours at 58 °C and deparaffinized with three xylene washes (5 min each), hydrated in absolute alcohol and washed in distilled water. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Antigen retrieval was performed by pressure-cooking for 3 min in citrate buffer (10 mmol/L, pH 6.0). After washing in distilled water and phosphate-buffered saline (PBS), sections were incubated with primary antibodies for 30 min at room temperature: anti-ACE (1:50), anti-Angio II (1:2000), anti-TGFβ1 (1:600). After the final PBS wash, the slides were incubated with the polymer reagent (EnVision-Dako Inc., Carpinteria, CA) for 30 min at room temperature and washed in PBS. The reaction was visualized with diaminobenzidine (DAB) (Dako Inc., Carpinteria, CA). Counterstaining was performed with 50% Harris hematoxylin and after washing in water, sections were mounted with Permount. Negative controls included omitting the primary antibody.
Semiquantitative analysis of ACE, Angio II and TGFβ1 immunostained slides were graded for ACE, Angio II and TGFβ1 expression within glomeruli, tubules, vessels and interstitial cells. Semiquantitative analysis of ACE, Angio II and TGFβ1 tissue expression in different renal structures was graded from 0 to 4: 0 negative staining, 1 + mild, 2 + moderate, 3 + intense and 4 + very intense. A total expression index for each marker was calculated by the sum of the grades in all structures of the renal biopsy and expressed as median (min–max).
Statistical analysis
All statistical calculations were performed using Sigma Stat, version 1.0 (Windows, Systat, San Jose, CA). Data are reported as the mean ± standard deviation (SD) or as the median with ranges.
CAN groups comparisons were analyzed using the Kruskal–Wallis complemented by Dunn method. We used the nonparametric Mann–Whitney U test for variables without normal distribution. Correlation between noncontinuous variables was calculated by Spearman's correlation coefficient.Citation18
Graft survival was calculated by the Kaplan–Meier method and the log-rank test using the GraphPad Prism Software, version 5.05 (GraphPad Prism Software Inc., San Diego, CA). A p value less than 0.05 was considered statistically significant. The study was approved by the Research Ethics Committee of the Botucatu School of Medicine (Of. 299/08).
Results
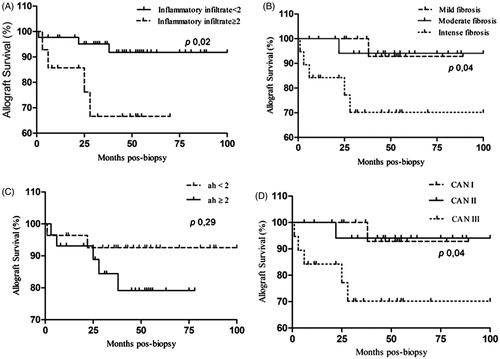
CAN group cases were graded as: grade I (22 cases), II (17 cases) and III (19). It was not observed transplant glomerulopathy in any case. Intimal arterial fibrosis was present in two biopsies. Hyaline arteriolar thickening was found in 45 biopsies: CAN I (ah1 = 5, ah2 = 6, ah3 = 6), CAN II (ah1 = 6, ah2 = 4, ah3 = 1), CAN III (ah1 = 4, ah2 = 10, ah3 = 3). The severity of hyaline arteriolar thickening was not significantly correlated with the CAN grade (p 0.15). Mononuclear inflammatory infiltrate was present in atrophic areas with tubular atrophy and interstitial fibrosis in 27 cases. The interstitial inflammatory infiltrate was significantly more frequent and severe in CAN grade III (CAN I = two cases of mild inflammatory infiltrate; CAN II = seven mild, one moderate; CAN III = four mild, five moderate and eight severe) (p < 0.001).
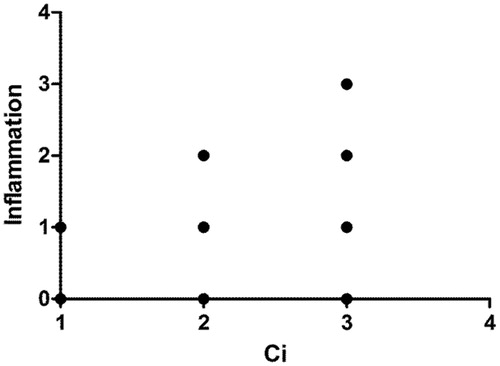
And the association of Spearman variables showed positive correlation between interstitial inflammatory infiltrate and interstitial fibrosis (r = 0.719, p < 0.0001) ().
Figure 1. Spearman's association between inflammatory infiltrate and interstitial fibrosis (Ci, Banff'score) (r = 0.719; p < 0.0001).
Characterization of intrarenal RAS
The presence of intrarenal ACE and Angio II was analyzed by immunohistochemistry.
Angiotensin converting-enzyme (ACE)
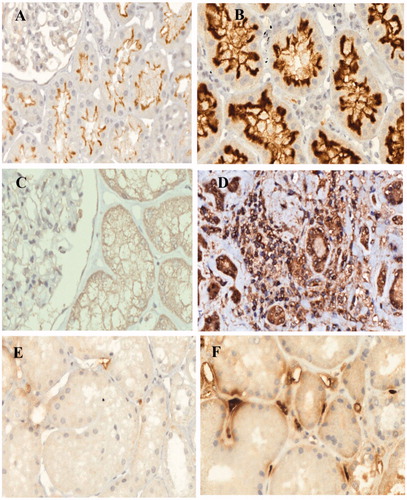
In the CAN group a marked increase of ACE staining was observed, in more preserved areas, in the brush border of proximal tubules in 55 biopsies, mesangial cells and podocytes in 43 biopsies and arterial endothelial cells in 3 biopsies; Spearman coefficient analysis showed statistically significant association between tubular scores and ACE staining; more differentiated tubules showed higher staining than atrophic tubules (r = −0.448, p < 0.001); in the control group we found weakly ACE immunostaining in the brush border of tubular cells (20 biopsies) and mesangial cells and podocytes (8 biopsies) ().
Figure 2. Intrarenal renin–angiotensin system and transforming growth factor β1. Angiotensin-converting enzyme (ACE): Control group (A)—mild staining of proximal tubules brush border; CAN group (B)—intense staining of proximal tubules brush border. Angiotensin II (Angio II): Control group (C)—negative; CAN group (D)—positive staining in atrophic tubules and interstitial cells. Transforming growth factor β1 (TGFβ1): Control group (E)—mild focal staining of peritubular capillaries; CAN group (F)—intense staining of peritubular capillaries. IH-400×.
Angiotensin II (Angio II)
All kidney biopsies of the control group were negative for Angio II. Angio II analysis was done in 57 biopsies of CAN. There was an increase in Angio II in tubular and endothelial cells from arterioles and arteries in 33 biopsies; positive expression of interstitial infiltrating cells was found in 27 biopsies. There were no significant differences of Angio II staining between differentiated and atrophic tubules (r = 0.243, p > 0.05) ().
Transforming growth factor β1
Transforming growth factor β1 (TGFβ1) immunohistochemical staining was performed in 55 biopsies. TGFβ1-positive cells were found in mesangium, tubules, mainly atrophic and dilated, and endothelial cells of arteries, arterioles and peritubular capillaries. There were no significant differences of TGFβ1 staining between differentiated and atrophic tubules (r = −0.159, p > 0.05). The peritubular capillaries staining was extensive in 26 biopsies with 26–50% of tissue involved; in 25 biopsies the area with stained capillaries was 11–25% and in 4 cases 1–10%.
In the control group, the TGFβ1 expression was mild and was present in glomeruli (20 biopsies), tubular cells (3 biopsies) and endothelial cells of arteries (15 biopsies) and peritubular capillaries (18 biopsies) ().
Correlations of ACE, Angio II and TGFβ1 expression in CAN and the control groups
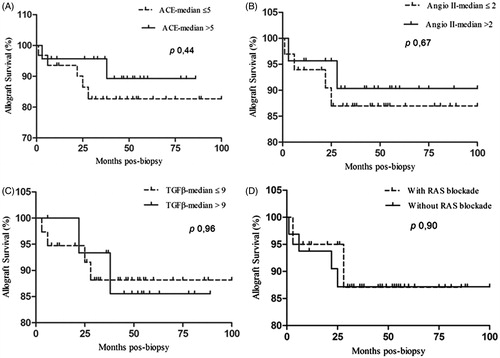
The Angio II and TGFβ1 median scores were significantly higher in the CAN (Angio II 0.0–7.0—median 2,0; TGFβ1 2.0–12.0—median 9.0) than the control group (Angio II 0.0–0.0—median 0.0; TGFβ1 1.0–7.0—median 5.0) (p < 0.001). No significant differences were observed between ACE staining scores of CAN and the control groups (CAN 1.0–9.0—median 5.0; control 3.0–8.0—median 4.0) (p 0.37). Among the CAN groups (CAN I, II and III) we observed statistically significant less expression of ACE in the CAN III. When we compare the biopsies of CAN with and without hyaline arteriolar thickening (Banff score ah), there were no significant differences with ACE (p 0.09), Angio II (p 0.28) and TGFβ1 (p 0.59) ().
Table 2. Angiotensin converting-enzyme (ACE), angiotensin II (Angio II) and transforming growth factor β1 (TGFβ1) expression in groups of chronic allograft nephropathy (CAN; CAN grades I, II and III; CAN with and without arteriolar hyaline thickening; CAN with or without RAS blockade) and the control group.
Spearman association showed negative correlation between inflammatory infiltrate (r = −0.471, p < 0.001) and interstitial fibrosis (r = −0.448, p < 0.001) with ACE scores; areas of inflammatory chronic changes showed less expression of ACE. There were no association of Angio II (r = 0.243; p < 0.068) and TGFβ1 expression (r = −0.159; p 0.24) with interstitial fibrosis by the Spearman coefficient analysis.
Clinico-pathological correlations
There were no significant differences of ACE, Angio II and TGFβ1 expression between the patients who were treated with RAS blockade and the patients who have not received this therapy ().
Angio II and TGFβ1 expression was not significantly different between the biopsies of the patients who lost their graft and the patients who have a functional allograft at the final follow-up.
The only morphological variable that showed correlation with a clinical finding was a positive correlation of TGFβ1 expression with previous acute rejection episodes (r = 0.331; p < 0.005). Serum creatinine at 12 months showed positive correlation with serum creatinine in the final follow-up (r = 0.296; p < 0.05).
Graft survival
Individual tubular atrophy, interstitial fibrosis and hyaline arteriolar thickening scores did not show statistical significant differences comparing patients with functional graft and patients who lost their graft (p = 0.062, 0.062 and 0.31, respectively).
There were no significant statistical correlation between ACE, Angio II and TGFβ1 expression with graft survival, and RAS blockade did not influence the graft outcome ().
Figure 3. Allograft survival after the diagnosis of CAN according to ACE, Angio II, TGFβ1 expression and RAS blockade.
The CAN III cases with severe interstitial fibrosis showed 70% survival when compared with 95% of CAN with mild and moderate fibrosis in 8 years (p 0.03). The group that had moderate or severe inflammatory infiltrate had worse survival (65%) than the group with absent or mild inflammatory infiltrate (95%) at 8 years (p 0.02). There was no statistical significant correlation between graft survival and hyaline arteriolar thickening (ah < 2 and ≥2) (p 0.29) ().
Discussion
We studied 58 post-transplant patients with gradual and progressive deterioration of renal function. To better find out the cases and study them, we maintain the generic term “CAN” now defined by Banff criteria as tubular atrophy and interstitial fibrosis without specific etiology (IFTANOS).Citation19 The causes and mechanisms of the chronic changes in the studied cases are unknown. Immunological and non-immunological injuries have been considered.Citation2,Citation20
Chronic rejection was ruled out because any of these cases showed chronic glomerulopathy with double contours and concentric intimal thickening of arteries; the C4d was negative in all the cases. In the long-term follow-up nonspecific tubulointerstitial damage (CAN) is more frequent (93.2%) than chronic rejection with specific glomerular and vascular changes (5.8%).Citation21
In this setting, we searched for local allograft expression of RAS components in the biopsies, correlated with clinico-histological findings and evaluated if there is an involvement of this system in the tissue injury.
RAS is stimulated following renal transplantation and intrarenal RAS may have significant effects in chronic allograft dysfunction. Experimental studies and clinical observations have been shown a role of RAS in CAN.Citation7,Citation22 Renal gene and protein expression of SRA components, mainly in tubulo-interstitial compartment show correlation with increased activity of growth factors and the severity of lesions.Citation14,Citation15 ACE inhibition and Angio II receptor blockers (ARBs) can slow the progressive injury in CAN.Citation10–12,Citation22
Our results indicate up-regulation of Angio II converting-enzyme and Angio II with high expression in various structures of the allograft in the CAN group. However, there were no significant association between Angio II tissue expression with CAN grades and the severity of interstitial fibrosis; ACE expression was lower in the atrophic areas. We did not find any correlation of ACE, Angio II and TGFβ1 expression with serum creatinine levels at 12 months, at the final follow-up and with graft outcome.
Oka et al.Citation13 studied renin expression by immunohistochemistry and did not find correlation with CAN grade and clinical data. Mas et al.Citation15 found a positive relationship of intragraft mRNA expression of angiotensinogen with TGF-β1 and high levels of proteinuria. Expression of mRNA type 2 Angio II receptor was significantly greater in patients with CAN and correlated with metalloproteinases and inhibitors of metalloproteinases, indicating a role in the extracellular matrix modulation.Citation14 These results cannot be correlated properly with our findings because they used different markers and methods.
In our cases the RAS blockade did not attenuate the tissue injury and did not result in increased graft survival. Some studies have been shown the renoprotective effects of angiotensin converting-enzyme inhibitors (ACEIs) and ARBs in CAN. Montanaro et al.Citation10 studying transplant recipients with good stable renal function, showed reduced proteinuria and increased creatinine clearance in patients who received RAS blockade. In the study of Zaltzman et al.Citation12 the patients who received RAS blockade therapy had a slow rate of chronic allograft dysfunction and had 83% of graft survival at five years. Artz et al.Citation11 found a median graft survival of 6.3 years in patients treated with RAS blockade as opposed to 1.8 years in the patients not treated. Experimental models of chronic allograft dysfunction with SRA blockade showed reduction of inflammatory cells and levels of cytokines ameliorating allograft lesions.Citation23–26
Although ACEIs or receptors antagonists have been shown some benefit in CAN, there is a consensus that more prospective large randomized studies are necessary to understand the renoprotective effect of RAS blockade after kidney transplantation.Citation4,Citation7 RAS blockade can reduce the chronic changes but is not sufficient to halt renal fibrosis.Citation27 Angio II is also synthesized by other alternative enzymatic pathways and a complete suppression of the RAS is not achieved by ACE inhibitors alone.Citation28
RAS is a mediator of calcineurin inhibitor toxicity through synthesis and up-regulation of TGFβ1 with development of fibrosis.Citation9 Forty-five (45) biopsies showed some grade of arteriolar hyaline thickening (ah scores) suggesting some component of calcineurin inhibitor toxicity as a cause of interstitial fibrosis and deterioration of renal function. Although we did not find significant correlation of ah scores with CAN grade and ACE, Angio II and TGFβ1 expression, we cannot totally rule out drug nephrotoxicity as a cause of renal fibrosis.
An important finding in this group of CAN cases was the lymphomononuclear inflammatory infiltrate present in 27 biopsies in areas of tubular atrophy and interstitial fibrosis. In CAN grade III, we observed in eight cases a heavy and active inflammatory infiltrate with tubulitis in atrophic tubules that not fulfilled criteria of acute rejection or infection. These chronic inflammatory processes did not show significant correlation with the expression of Angio II. These findings do not support a role of the RAS in the inflammatory process present in these cases. We observed a high percentage (43.1%) of acute cellular rejection episodes that showed a positive correlation with TGFβ1 expression (r = 0.331; p < 0.05). Previous acute cellular rejection episodes are highly predictive of chronic damage.Citation3,Citation29 From 23 patients who had previous acute rejection episodes, 10 (43.5%) showed inflammatory infiltrate in scarred areas and 8.7% lost their graft.
Inflammatory cells in chronic areas are not carefully analyzed in the literature. Tubulitis in atrophic tubules and inflammatory infiltrate in areas of interstitial fibrosis are considered unspecific findings by Banff criteria.Citation17 Transplant glomerulopathy and chronic allograft arteriopathy are the only pathologic features that are attributed to alloimmune mechanisms.Citation19
Usually, inflammatory activity is minimal in CAN and some studies suggest that the presence of mild tubulitis is not a significant factor determining graft outcome.Citation17,Citation21,Citation30 More recently, some studies report the combination of tubular atrophy and interstitial fibrosis with inflammation associated with late graft loss.Citation20,Citation31–34 However, these are studies of protocol biopsies detecting subclinical rejection associated with CAN and that have a poor graft outcome.Citation33–34 But, in patients with slow deterioration of renal function with a biopsy-proven CAN, the presence of interstitial inflammatory infiltrate and tubulitis restrict to atrophic tubules is interpreted as non-specific finding. The diagnosis of these cases is difficult and treatment options are unknown. A few studiesCitation34–36 suggested that this unspecific inflammatory infiltrate in scarred areas left untreated, insidiously destroys tubules with ongoing injury with interstitial matrix deposition and progressive allograft dysfunction.
Based on the best results of good HLA compatibility, utilization of living donors, protection from reperfusion injury that activate immunological response and good immunosuppression with reduction of acute rejection episodes, PonticelliCitation37 believes that the most important risk factor of long-term graft damage is the maintenance of immunological injury. The 9th Banff ConferenceCitation38 introduced a new optional score of a total inflammation in scarred and unscarred areas in renal allograft (ti) for the future evaluation.
In conclusion, our study do not confirm the involvement of RAS system in the CAN; local renal RAS expression did not correlate with the different grades of CAN and with graft survival; RAS blockade also did not improve tissue injury characterized mainly by tubular atrophy, inflammatory infiltrate and interstitial fibrosis. The most impressive morphological finding was the presence of inflammatory infiltrate in scarred areas that should be responsible for the chronic progressive changes. A high percentage of previous acute rejection episodes in these patients that correlate with TGFβ1 expression, and the positive association of inflammatory infiltrate with interstitial fibrosis suggest an ongoing immunological injury.
Acknowledgements
The authors thank Professor Carlos Roberto Padovani for the statistical analysis. The Abstract was submitted to Congress of Nephrology (XVII Congreso SLANH 2014).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Paul LC. Chronic allograft nephropathy: An update. Kidney Intern. 1999;56:783–793
- Joosten SA, Sijpkens WJ, Kooten CV, Paul LC. Rejeição crônica ao aloenxerto renal: Considerações fisiopatológicas. Kidney Intern. 2005;1:119–130
- Womer KL, Vella JP, Sayegh MH. Chronic allograft dysfunction: Mechanisms and new approaches to therapy. Semin Nephrol. 2000;20(2):126–147
- Waller JR, Nicholson ML. Molecular mechanisms of renal allograft fibrosis. Brit J Surg. 2001;88:1429–1441
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Rupérez M, Esteban V, Egido J. Inflammation and angiotensin II. Intern J Biochem Cell Biol. 2003;35:881–900
- Wolf G. Lesão renal devido à ativação do sistema renina-angiotensina-aldosterona pela via do fator de crescimento transformador b. Kidney Intern. 2007;2:129–133
- Geara AS, Azzi J, Jurewicz M, Abdi R. The renin–angiotensin system: An old, newly discovered player in immunoregulation. Transplant Rev. 2009;23:151–158
- Campistol JM, Inigo P, Larios S, Bescos M, Oppenheimer F. Role of transforming growth factor-b-1 in the progression of chronic allograft nephropathy. Nephrol Dial Transplant. 2001;16(Suppl 1):114–116
- Shang M-H, Yuan W-J, Zhang S-J, Fan Y, Zhang Z. Intrarenal activation of renin angiotensin system in the development of cyclosporine A induced chronic nephrotoxicity. Chin Med J. 2008;121(11):983–988
- Montanaro D, Gropuzzo M, Tulissi P, et al. Renoprotective effect of early inhibition of the renin–angiotensin system in renal transplant recipients. Transplant Proc. 2005;37:991–993
- Artz MA, Hilbrands LB, Borm G, Assmann KJM, Wetzels JFM. Blockade of the renin–angiotensin system increases graft survival in patients with chronic allograft nephropathy. Nephrol Dial Transplant. 2004;19:2852–2857
- Zaltzman JS, Nash N, Chiu R, Prasad GVR. Renin–angiotensin system blockade in biopsy-proven allograft nephropathy. Transplant Proc. 2003;35:2415–2417
- Oka K, Moriyama T, Takahara S, et al. Increased expression of renin in chronic allograft nephropathy. Transplant Proc. 2005;37:2131–2134
- Becker BN, Jacobson LM, Hullett DA, et al. Type 2 angiotensin receptor expression in human renal allografts: An association with chronic allograft nephropathy. Clin Nephrol. 2002;57:19–26
- Mas V, Alvarellos T, Giraudo C, Massari P, Boccardo G. Intragraft messenger RNA expression of angiotensinogen: Relationship with transforming growth factor beta-1 and chronic allograft nephropathy in kidney transplant patients. Transplantation. 2002;74(5):718–721
- Mas V, Maluf D, Archer K, et al. Establishing the molecular pathways involved in chronic allograft nephropathy for testing new noninvasive diagnostic markers. Transplantation. 2007;83:448–457
- Racusen LC, Solez K, Colvin RB, et al. The Banff working classification of renal allograft pathology. Kidney Intern. 1999;55:713–723
- Zar JH. Biostatistical Analysis. New Jersey, USA: Prentice-Hall; 1999
- Solez K, Colvin RB, Racusen LC, et al. Banff'05 meeting report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (“CAN”). Am J Transplant. 2007;7:518–526
- Arias M, Séron D, Moreso F, Bestard O, Praga M. Chronic renal allograft damage: Existing challenges. Transplantation. 2011;91(9S):S4–S25
- Nankivel BJ, Borrows RJ, Fung CLS, O'Connell PJ, Allen RDM, Chapman JR. The natural history of chronic allograft nephropathy. New Eng J Med. 2003;349:2326–2333
- Moscoso-Solorzano GT, Mastroiani-Kirsztajn G, Ozaki KS, Franco MF, Pacheco-Silva A, Câmara NOS. Synergistic effect of mycophenolate mofetil and angiotensin-converting enzyme inhibitor in patients with chronic allograft nephropathy. Brazil J Med Biol Res. 2009;42:445–452
- Noris M, Mister M, Pezzotta A, et al. ACE inhibition limits chronic injury of kidney transplant even with treatment started when lesions are established. Kidney Intern. 2003;64:2253–2261
- Smit-van Oosten A, Henning RH, Goor HV. Strain differences in angiotensin-converting enzyme and angiotensin II type I receptor expression. Possible implications for experimental chronic renal transplant failure. J RAAS. 2002;3(1):46–53
- Szabo A, Lutz J, Schleimer K, et al. Effect of angiotensin-converting enzyme inhibition on growth factor mRNA in chronic renal allograft rejection in the rat. Kidney Intern. 2000;57:982–991
- Ziai F, Nagano H, Kusaka M, et al. Renal allograft protection with losartan in Fisher → Lewis rats: Hemodynamics, macrophages, and cytokines. Kidney Intern. 2000;57:2618–2625
- Border WA, Noble NA. Interactions of transforming growth factor-b and angiotensin II in renal fibrosis. Hypertension. 1998;31(Part 2):181–188
- Cheng ZJ, Vapaatalo H, Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit. 2005;11(6):RA194–RA205
- Harris S, Coupes BM, Roberts SA, Roberts IS, Short CD, Brenchley PE. TGFβ1 in chronic allograft nephropathy following renal transplantation. J Nephrol. 2007;20(2):177–185
- Seron D, Moreso F, Bover J, et al. Early protocol renal allograft biopsies and graft outcome. Kidney Intern. 1997;51:310–316
- Mengel M, Chapman JR, Cosio FG, et al. Protocol biopsies in renal transplantation: Insights into patient management and pathogenesis. Am J Transplant. 2007;7:512–517
- Mengel M, Gwinner W, Schwarz A, et al. Infiltrates in protocols biopsies from renal allografts. Am J Transplant. 2007;7:356–365
- Moreso F, Ibernon M, Goma M, et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Tranplant. 2006;6:747–752
- Shishido S, Asanuma H, Nakai H, et al. The impact of repeated subclinical acute rejection on the progression of chronic allograft nephropathy. J Am Soc Nephrol. 2003;14:1046–1052
- Divella C, Rossini M, Loverre A, et al. Immunohistochemical characterization of glomerular and tubulointerstitial infiltrates in renal transplant patients with chronic allograft dysfunction. Nephrol Dial Transplant. 2010;25:4071–4077
- Chapman JR, O'Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005;16:3015–3026
- Ponticelli C. Progression of renal damage in chronic rejection. Kidney Intern. 2000;57(Suppl. 75):S62–S70
- Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–760