Abstract
Objective: The aim of our study was to determine the amount and composition of immune cells within glomeruli and PTCs and its relationship with C4d deposition. Materials and methods: Immunohistochemistry staining for C4d, CD3, CD68, granzyme B and Foxp3 was used for phenotyping and enumerating immune cells within intracapillaries. Results: C4d staining was present in 26 biopsy specimens (C4d+) and negative in 25 specimens (C4d−). The total number of infiltrating cells in glomerulus and PTC in C4d+ was significantly higher than in C4d−. Although the C4d+ showed a significantly higher mean number of macrophages per glomerulus and PTC than in C4d− group, the C4d− showed a higher mean number of T cells per glomerulus and PTC than in C4d+. Comparing cell counts in diffuse C4d+ and focal C4d+ groups, a significant difference of absolute numbers of intracapillary cells could be observed in glomeruli and PTCs. The mean number of macrophages per glomerulus and PTC in diffuse C4d+ was greater than that of the focal C4d+, while mean T cells per glomerulus and PTC were less in cases of diffuse C4d+ than in focal C4d+. The differences, however, did not achieve statistical significance. Not only glomerular T cells but also PTCs are granzyme B positive T cells totally. Conclusion: The total number of infiltrating cells in glomeruli and PTC has association with PTC C4d deposition; the infiltrating cells were predominantly macrophages in C4d+, especially in diffuse C4d+, whereas the infiltrating cells were predominantly T cells in C4d−. Glomerular and PTC T cells were cytotoxic phenotype completely.
Introduction
Allograft glomerulitis and peritubular capillaritis (PTCitis) have been correlated with C4d deposition and antibody-mediated rejection and with an increased probability of graft loss.Citation1
Inflammatory cells within glomerular and peritubular capillaries (PTCs) are a common morphological finding in allograft rejection. Glomerulitis and PTCitis characteristic with mononuclear cell infiltration of glomerular and PTCs are a well-recognized component of the acute allograft rejection reaction and are observed in a proportion of biopsy specimens showing acute rejection (AR).Citation2–4 Both T cells and macrophages have been shown in glomerular and PTCs in allografts with transplant glomerulitis and PTCitis.Citation4–6 Recent studies suggested an association of allograft glomerulitis and PTCitis with acute humoral rejection (AHR),Citation7 PTC C4d deposition.Citation6,Citation8,Citation9
Complement split factor C4d is produced by activation of the classical complement pathway by antigen–antibody complexes and binds covalently to tissue elements at the site of activation. Deposition of C4d along PTC has closely related to circulating donor-specific antibody levels in renal allograft recipients experiencing AR and has been confirmed as a marker for AHR.Citation10–13 Strong diffuse (involving > 50% of PTC) staining of PTC for C4d has been included as one of the criteria for the diagnosis of AHR in a recent update of the Banff '97 classification of renal allograft rejection.Citation14 However, the significance of focal PTC C4d deposition is controversial.Citation13 Magil et al.Citation4,Citation6 discovered that glomerular and PTC macrophages have been shown to be the predominant cell type in transplant glomerulitis and PTCitis in biopsy specimens showing AR with PTC C4d deposition, whereas in C4d negative biopsies which is mainly composed of T cells. Previous studies have confirmed that Granzyme B, a protein released by cytotoxic T lymphocytes, is associated with AR.Citation15–19 But did not note any significant differences about the cell types between diffuse C4d positive with focal C4d positive transplant glomerulitis and PTCitis. Similarly, also did not find whether there are cytotoxic T cells or regulatory T cells or both.
Objectives
The aim of our study was therefore to quantify and immunohistochemically characterize the glomeruli and PTC cells in different types of rejection: first, to determine whether accumulation of mononuclear cells in glomeruli and PTC is truly distinctive in different rejection; second, whether in glomeruli and PTC immune cell population, focal C4d-positive rejection is different from that in cases with diffuse C4d-positive rejection and third, whether in glomeruli and PTC, T-cell phenotype is in different rejection and different extent of C4d-positive rejection.
Materials and methods
Patients
Only patients who underwent biopsy within 3–30 days of transplantation between 1 June 2006 and 30 June 2012, and whose allograft biopsy specimens showed both AR according to the Banff '97 criteriaCitation20 and transplant glomerulitis and PTCitis and who had paraffin tissue for immunohistochemistry for C4d, CD3, CD68, granzyme B and Foxp3 were included in this study. Fifty-one biopsy specimens from 51 patients (29 males, 22 females) met selection criteria. It was done after the approval of the Ethic Committee at the Zhe Jiang Provincial People's Hospital and the consent of patients.
Histopathology
All biopsy specimens were formalin fixed (10%, PH 7.2) and paraffin embedded according to the standard procedures. For histopathologic analysis, 2 μm sections were stained with H&E, PAS, Trichrome-stain and Jones's Methenamin Silver.Citation21 Allograft rejection was assessed and graded according to the Banff classification of renal allograft rejection.Citation20
Immunohistochemical examination
Immunohistochemistry was performed on formalin fixed tissue embedded in paraffin. After dewaxing in xylol, endogenous peroxidase activity was blocked with 0.03% H2O2. Antigen retrieval was performed using 0.1 M citrate buffer pH 6.0 under pressure, and non-specific reactivity was blocked with normal horse serum. The primary antibodies were rabbit polyclonal antibodies specific for complement split factor C4d (Purchased from Biomedical Corporation, Vienna, Austria), mouse monoclonal antibodies specific for CD3 (a marker for T cells; Clone: 5B10; Dako, Glostrup, Denmark), CD68 (a marker for macrophages; Clone: 236A/E7; Dako), granzyme B (a marker for cytotoxic T cells; Clone: 246A; CHEMICON, Temecula, CA) and Foxp3 (a marker for regulatory T cells; Clone: 36B; Abcam, Cambridge, UK) and were used at a dilution of 1:100. The sections were incubated with the primary antibody at 4 °C overnight. Sections were then incubated for 30 min at room temperature in a proprietary polymer-based secondary antibody (Dako Envision Plus), and then stained with diaminobenzidine (Dako) for 6 MiniSlide were washed with phosphate buffered saline between steps. Sections were counterstained in Harris's hematoxylin for 2 min, rinsed in running tap water for 5 min, dehydrated and mounted with Hystomount. Lymph node tissue was used as positive controls for CD68, CD3, granzyme B and Foxp3. Negative control studies were performed by omitting the primary monoclonal antibody in the staining procedure and using an irrelevant mouse monoclonal antibody as the primary antibody. The criteria for C4d-positive are as our previously described.Citation22
Scoring of C4d staining (% of biopsy or five high-power fields)Citation23
C4d0: Negative: 0%.
C4d1: Minimal C4d stain/detection: < 10%.
C4d2: Focal C4d stain/positive: 10–50%.
C4d3: Diffuse C4d stain/positive: > 50%.
Quantitative analysis
All biopsies were scored according to the Banff '97 criteriaCitation20 to determine the type and grade of the rejection reaction. The number of macrophages, T cells, granzyme B positive T cells and Foxp3 positive T cells in glomeruli with full cross-sectioned profiles were counted in each biopsy specimen and expressed as the number of macrophages, T cells, granzyme B positive T cells and Foxp3 positive T cells per glomerulus. The number of macrophages, T cells, granzyme B positive T cells and Foxp3 positive T cells in PTCitis in at least six consecutive HPF on sections and expressed as the number of macrophages, T cells, granzyme B positive T cells and Foxp3 positive T cells per PTC. In addition, the glomerular, PTCs monocyte_T cell biopsy specimens were divided into two groups according to whether they showed diffuse or focal PTC staining for C4d (C4d+ group) or not (C4d− group). Mean numbers (±SD) of macrophages, T cells, granzyme B positive T cells and Foxp3 positive T cells per glomerulus and PTC were determined for each group. Mean numbers (±SD) of macrophages, T cells, granzyme B positive T cells and Foxp3 positive T cells per glomerulus and PTC were also determined for diffuse or focal PTC staining for C4d, respectively. Counting was performed independently by two observers blinded to the results of C4d staining and mean values of the two readings were used for calculations.
Statistical analysis
Results are expressed as the mean ± SD. Chi-square test, Fisher exact test, one-way ANOVA and Kruskal–Wallis were used for comparisons between groups. All p-values were two-tailed and a p-value < 0.05 was considered significant.
Results
Patient data and histological features
The characteristics of the two groups are presented in . Fifty-one patients (22 males, 29 females) had 51 biopsy specimens that showed AR and transplant glomerulitis and PTCitis. Twenty biopsy specimens from 12 males and 14 females showed diffuse (n = 14) or focal (n = 12) PTC C4d staining (C4d+ group), whereas 25 biopsy specimens from 10 males and 15 females were negative for PTC C4d (C4d− group). In the C4d+ group, at least 10 glomeruli were available for histological examination in 20 biopsy specimens. In the other six C4d+ biopsy specimens, nine glomeruli were present in four specimens and eight glomeruli were noted in the other two specimens. In the C4d− group, all except five biopsy specimens contained at least 10 glomeruli. The other biopsy specimens had nine glomeruli each. All biopsy specimens had at least two interlobular arteries. Distribution of grades of rejection for the C4d+ group (1A, n = Banff15; 1B, n = 2; and 2A, n = 9) was not significantly different from that of the C4d− group (1A, n = 14; 1B, n = 1; and 2A, n = 10). There was no significant difference between mean times for biopsy post-transplantation for the C4d+ (12.0 ± 8.8) and C4d− biopsy specimens (9.8 ± 7.6). Also, no statistically significant differences were observed between two groups for age, gender, duration of cold or warm ischemia, HLA mismatches, complement-dependent-cytotoxic (CDC) result, panel reactive antibody (PRA) > 10% pretransplantation, Biopsy time post-transplantation, serum creatinine values on biopsy time and immunosuppression protocol.
Table 1. Demographic date and clinical characteristics of patients.
Total number of inflammatory cells in glomeruli and PTCs is distinctive for C4d positive and C4d negative biopsies
Quantitative results are listed in . Biopsy specimens showed varying numbers of macrophages and T cells in glomerulus and PTC ( and ). An estimate of the total numbers of glomerulus and PTC cells was determined by summing up the counts for CD3 and CD68 positive cells within glomeruli and PTCs. Comparing cell counts in C4d− and C4d+ glomerulitis and PTCitis biopsies, a significant difference of absolute numbers of intracapillary cells could be observed in glomeruli and PTCs (). The total number of inflammatory cells in glomeruli and PTCs is significantly higher in C4d+ biopsies than in C4d− biopsies (p < 0.0001; p < 0.0001).
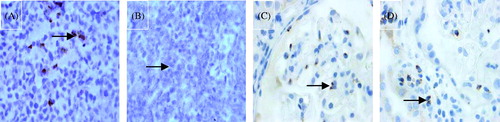
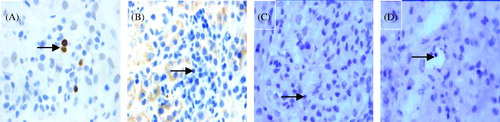
Figure 1. Example of the glomerulitis and immunostaining for two markers (×400, DAB stain × 400): (A) glomerulitis with HE stain (arrow); (B) CD3-positive controls; (C) CD3-negative controls; (D) CD3-positive T lymphocytes in the glomerulus (arrow); (E) glomerulitis with PAS stain (arrow); (F) CD68-positive controls. (G) CD68-negative controls; (H) CD68-positive T lymphocytes in the glomerulus (arrow).
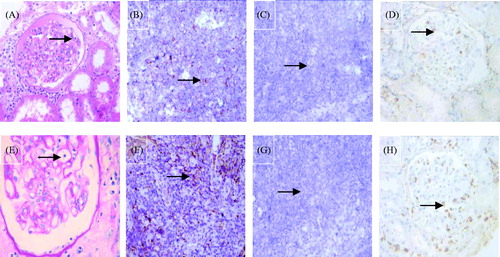
Figure 2. Example of the two PTCitis scores immunostaining for two markers (PAS stain × 1000, DAB stain × 1000). (A) Capillaritis with a PTCitis-score 1, i.e., max 3–4 luminal inflammatory cells in PTC (arrow). (B) Capillaritis with a PTCitis-score 2, i.e., max 5–10 luminal inflammatory cells in PTC (arrow). (C) CD3-positive T lymphocytes in the PTC (arrow). (D) CD68-positive macrophages in the PTC (arrow).
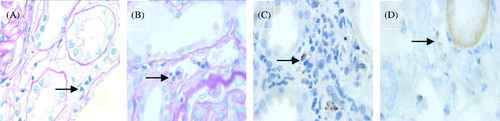
Figure 3. Immunostaining for C4d (DAB stain × 1000). (A) Negative C4d stain in PTC (arrow). (B) Minimal C4d stain/positive in PTC (arrow), i.e., C4d deposition in < 10% PTCs. (C) Focal C4d stain/positive in PTC (arrow), i.e., C4d deposition in 10–50% PTCs. (D) Diffuse C4d stain/positive in PTC (arrow), i.e., C4d deposition in > 50% PTCs.
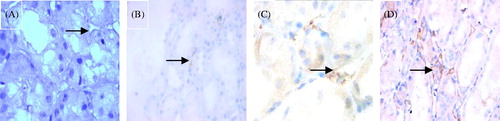
Table 2. Quantitative analysis of biopsy findings for C4d+ and C4d− groups.
Endocapillary macrophages predominate in C4d positive biopsies
Comparing the numbers of CD3-positive and CD68-positive cells within glomeruli and PTCs (), we found that the number of glomerular and PTC macrophages was higher in cases of C4d+ (13.73 ± 7.03; 4.36 ± 1.85) than in C4d− group (2.57 ± 1.22; 2.26 ± 1.64) (p < 0.0001; p = 0.001). However, the number of glomerular and PTC T cells was less in cases of C4d+ (4.05 ± 2.60; 1.29 ± 0.52) than in C4d− group (5.60 ± 2.81; 2.01 ± 1.02) (p = 0.023; 0.031). It is interesting that not only glomerular T cells but also PTC T cells are all granzyme B positive T cells but no one Foxp3 positive T cells ( and ). The number of glomerular and PTC granzyme B positive T cells was less in cases of C4d+ (3.37 ± 2.34; 1.01 ± 0.58) than in C4d− group (4.27 ± 2.41; 1.98 ± 0.96). The differences, however, did not achieve statistical significance (p = 0.141; p = 0.231).
Endocapillary macrophages are higher in diffuse C4d positive group than that of focal C4d positive group
The C4d+ biopsies can be divided into two subgroups based on whether there was a diffuse strong or focal moderately strong or strong PTC staining for C4d. Twenty-three biopsies from 11 patients biopsied had a diffuse PTC C4d reaction, 14 biopsies showed focal PTC C4d staining. Comparing cell counts in diffuse C4d+ and focal C4d+ groups, a significant difference of absolute numbers of intracapillary cells could be observed in glomeruli and PTC () (23.25 ± 4.71 vs. 14.49 ± 6.86, p = 0.001; 7.53 ± 2.38 vs. 4.32 ± 1.89, p < 0.0001). The extent of glomerular and PTC infiltration by macrophages as expressed by the mean number of macrophages per glomerulus and PTC in the diffuse C4d+ group (19.62 ± 4.97; 5.89 ± 2.03) was significantly greater than that of the focal C4d+ group (9.11 ± 4.48; 2.67 ± 1.73) (p < 0.0001; p = 0.001). The number of glomerular and PTC T cells was less in cases of diffuse C4d+ (3.64 ± 1.50; 2.97 ± 0.43) than in focal C4d+ group (4.38 ± 3.24; 2.36 ± 0.98). The differences, however, did not achieve statistical significance (p = 0.74; p = 0.53). Similarly, glomerular and PTC T cells are all granzyme B positive T cells but no one Foxp3 positive T cells. The number of glomerular and PTC granzyme B positive T cells was less in cases of diffuse C4d+ (2.99 ± 1.36; 1.31 ± 0.45) than in focal C4d+ group (4.57 ± 2.21; 2.04 ± 0.76). The differences, however, did not achieve statistical significance (p = 0.215; p = 0.182).
Table 3. Quantitative analysis of biopsy findings for the diffuse C4d and focal C4d groups.
Discussion
Renal transplantation is the best form of renal replacement therapy for patients with end stage renal disease resulting in improved survival compared to chronic dialysis treatment. With the continuous advances in immunosuppressive therapy and prophylaxis of infectious complications, there is great improvement of early allograft survival and the long-term survival of renal allografts has improved significantly. AR still remains a problem following kidney transplantation because it is strongly associated with late transplant failure.Citation24,Citation25
This is the first study to quantify the relative numbers of glomerular and PTC T cells, macrophages, granzyme B and regulatory T cells in biopsy specimens with transplant glomerulitis and PTCitis and relate the results to PTC C4d deposition level. Early investigations showed both T cells and macrophages in glomeruli and PTCs in specimens, a predominantly macrophages infiltrate in specimens of transplant glomerulitis and PTCitis were associated with PTC C4d deposition, whereas T cells were predominant in specimens of transplant glomerulitis and PTCistis in PTC C4d− cases.Citation4,Citation6 As above-mentioned study combined the focal and diffuse C4d+ patients into one group for outcome analysis, no comparison of results with respect to outcome can be made. In present investigation, a predominantly macrophages infiltrate in specimens of transplant glomerulitis and PTCitis were associated with PTC C4d deposition, T cells were predominant in specimens of transplant glomerulitis and PTCitis in PTC C4d− cases, in agreement with previous reports. Whereas mean number of total cells per glomerulus and PTC and mean number of macrophages per glomerulus and PTC in diffuse C4d+ were higher than in the focal C4d+ group.
A series of studies have elucidated that Granzyme B, proteins released by cytotoxic T lymphocytes, is associated with AR. In contrast, a specialized subset of CD4+CD25+T lymphocytes (Treg cells) seems to suppress autoimmunity and maintain self-tolerance. Noninvasive measurements of urinary Foxp3 mRNA seem to predict the outcome of AR. We studied the immunophenotype of T lymphocytes infiltrating renal biopsy specimens of allografts AR. Our previously study confirmed that the ratio of infiltrating Granzyme B-positive to Foxp3-positive T cells was a sensitive, specific marker distinguishing a “cytotoxic phenotype” from a “regulatory phenotype”. Also, 85.9% of control biopsy specimens presented regulatory phenotypes. However, 96.2% and 92.0% of C4d+ AR and C4d− AR biopsy specimens presented the cytotoxic phenotype, respectively.Citation19 In this research, we found glomerular and PTC T cells are all granzyme B positive T cells but no one Foxp3 positive T cells. The number of glomerular and PTC granzyme B positive T cells were less in cases of diffuse than in focal C4d+ group.
Transplant glomerulitis and PTCitis have been associated with AHRCitation7 and PTC C4d depositionCitation6,Citation8,Citation9 which has been suggested as a marker of AHR.Citation10–13 It is tempting to consider transplant glomerulitis and PTCitis as a possible surrogate marker for AHR. However, as shown in this investigation, without knowledge of glomerular and PTC cell types and their relative numbers, this may be misleading. In view of the close correlation of glomerular and PTC macrophages with PTC C4d deposition and the predominance of glomerular T cells in transplant glomerulitis and PTCistis in PTC C4d− biopsy specimens, one might consider predominantly monocytic transplant glomerulitis and PTCitis as a marker for AHR. Furthermore, it should be noted that the total number of inflammatory cells in glomeruli and PTCs is distinctive between C4d+ and C4d− groups.
The focus of current immunosuppression is prevention of T-cell activation and infiltration into the transplanted organ.Citation26 The immunophenotype of the inflammatory cells in AR has long been thought to be predominantly T lymphocytic.Citation27 However, early studies using cellular markers in AR identified macrophages as a significant component of the mononuclear infiltrate in tubulointerstitial rejection.Citation12
While it has long been known that the cellular infiltrate in the tubulointerstitium of AR is predominantly composed of T cells,Citation28 it is widely recognized that macrophages are also involved in tubulitis, contributing to 38–60% of the inflammatory infiltrate.Citation29 It has been confirmed by many that the humoral immune has an important role in allograft rejection. Deposition of C4d along PTC has been suggested as a marker for AHR. Several studies also have shown a significant association between PTC C4d deposition and vascular rejection.Citation29–31 Wenqing et al.Citation32 discovered that 77.8% acute vacular rejection with C4d deposition along PTC. One previous study noted that the highest numbers of glomerular macrophage were found in cases of vascular rejection.Citation33 Another study showed that the macrophage is the predominant cell type in renal allograft intimal arteritis from acute vacular rejection biopsies which confirmed that the macrophage has relation with AHR.Citation34
The finding of glomerulitis and PTCitis predominantly composed of macrophages provides some insight into potential mechanisms of AR, which has largely been identified as a T-cell derived entity. It may be that only rare T lymphocytes in the subendothelium, or even T cells in the tubulointerstitium, are sufficient to induce glomerulitis PTCitis which is then largely composed of macrophages. Macrophages may contribute to AR and tissue damage by a number of mechanisms, including cell-mediated cytotoxicity,Citation35 antigen presentation and T-cell activation,Citation36 production of nitric oxide,Citation37 and release of inflammatory cytokines.Citation38 Finally, macrophages may induce myofibroblast proliferation through production of profibrogenic growth factors, leading to interstitial fibrosis and the occurrence of chronic allograft nephropathy.Citation39
Macrophages are known to be mediate tissue damage not only in the kidney but also in other organs. There is a growing evidence of their accumulation in graft rejection.Citation40 In a research using liposomal clodronate to deplete macrophages in which lymphocyte infiltration within the renal allograft was maintained, tissue damage was reduced and renal function was preserved. This shows an important role for the macrophage in graft destruction in AR.Citation41 This finding confirms that the mechanisms related to injury in AR are not only due to T-cell-mediated cytotoxicity but also may be due, at least in part, to injury derived from macrophages.
The prognostic significance of transplant glomerulitis and PTCitis is uncertain. One previous study discovered that the presence of transplant glomerulitis and PTCistis in biopsy specimens with borderline AR predicted progression to histological AR.Citation42
In conclusion, we provide evidence that humoral injury (indicated by endothelial C4d deposits in PTC) is associated with a predominantly macrophage infiltration within glomeruli and PTCs, especially in diffuse C4d deposits in PTC, furthermore, not only glomerular but also PTC T cells are all granzyme B positive T cells but no one Foxp3 positive T cells. In view of the association of transplant glomerulitis and PTCitis with PTC C4d deposition, transplant glomerulitis and PTCitis, especially the macrophage-predominant type, may have a negative impact on graft survival.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was funded by National Natural Science Foundation (81170691) and Provincial Foundation Project (WKJ 2013-2-002).
References
- Lefaucheur C, Nochy D, Hill GS, et al. Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant. 2007;7(4):832–841
- Marcussen N, Solez K, Spencer E, et al. Early transplant glomerulitis: Glomerular size and ultrastructure. Transplant Proc. 1996;28:468–469
- Olsen S, Spencer E, Cockfield S, et al. Endocapillary glomerulitis in the renal allograft. Transplantation. 1995;59:1421–1425
- Fahim T, Bohmig GA, Exner M, et al. The cellular lesion of humoral rejection: Predominant recruitment of monocytes to peritubular and glomerular capillaries. Am J Transplant. 2007;7:385–393
- Bishop GA, Hall BM, Duggin GG, et al. Immunopathology of renal allograft rejection analyzed with monoclonal antibodies to mononuclear cell markers. Kidney Int. 1986;29:708–717
- Magil AB, Tinckam K. Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int. 2003;63:1888–1893
- Trpkov K, Campbell P, Pazderka F, et al. Pathologic features of acute renal allograft rejection associated with donor-specific antibody. Transplantation. 1996;61:1586–1592
- Nickeleit V, Zeiler M, Gudat F, et al. Detection of the complement degradation product C4d in renal allografts: Diagnostic and therapeutic implications. J Am Soc Nephrol. 2002;13:242–251
- Sund S, Hovig T, Reisaeter AV, et al. Complement activation in early protocol kidney graft biopsies after living-donor transplantation. Transplantation. 2003;75:1204–1213
- Collins AB, Schneeberger EE, Pascual MA, et al. Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–2214
- Mauiyeddi SA, Crespo M, Collins AB, et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779–787
- Böhmig GA, Exner M, Habicht A, et al. Capillary C4d deposition in kidney allografts: A specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091–1099
- Feucht HE. Complement C4d in graft capillaries – The missing link in the recognition of humoral alloreactivity. Am J Transplant. 2003;3:646–652
- Racusen LC, Colvin RC, Solez K, et al. Antibodymediated rejection criteria – An addition to the Banff '97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714
- Mauiyyedi S, Crespo M, Collins AB, et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779–785
- Wevwer PC, Aten J, Rentenaar RJ, et al. Apoptotic tubular cell death during acute renal allograft rejection. Clin Nephrol. 1998;49:28–34
- Vasconcellos LM, Schachter AD, Zheng XX, et al. Cytotoxic lymphocyte gene expression in peripheral blood leukocytes correlates with rejecting renal allografts. Transplantation. 1998;66:562–566
- Li B, Hartono C, Ding R, et al. Noninvasive diagnosis of renal allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 2001;344:947–954
- Jin J, Xu Y, Wang H, et al. Peritubular capillaritis in early renal allograft dysfunction is an indicator of acute rejection. Transplant Proc. 2013;45:163–171
- Racusen L, Solez K, Colvin R, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723
- Grimbert P, Mansour H, Desvaux D, et al. The regulatory/cytotoxic graft-infiltrating T cells differentiate renal allograft borderline change from acute rejection. Transplantation. 2007;83:341–346
- Jianghua C, Wenqing X, Huiping W, et al. C4d as a significant predictor for humoral rejection in renal allografts. Clin Transplant. 2005;19:785–791
- Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760
- Hariharan S, McBride MA, Cherikh WS, et al. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62:311–316
- Meier-Kriesche HU, Schold JD, Kaplan B, et al. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289–1295
- Kaufman DB, Shapiro R, Lucey MR, et al. Immunosuppression: Practice and trends. Am J Transplant. 2004;4:38–53
- Underhill DM, Bassetti M, Rudensky A, et al. Dynamic interactions of macrophages with T cells during antigen presentation. J Exp Med. 1999;190:1909–1914
- Bishop GA, Hall BM, Duggin GG, et al. Immunopathology of renal allograft rejection analyzed with monoclonal antibodies to mononuclear cell markers. Kidney Int. 1986;29:708–717
- Herzenberg AM, Gill JS, Djurdjev O, et al. C4d deposition in acute rejection: An independent long-term prognostic factor. J Am Soc Nephrol. 2002;13:234–241
- Feucht HE, Schneeberger H, Hillebrand G, et al. Capillary deposition of C4d complement fragment and early graft loss. Kidney Int. 2003;43:1333–1338
- Kluth-Pepper B, Schneeberger H, Lederer SR, et al. Impact of human alloreactivity on the survival of renal allografts. Transplant Proc. 1998;30:1772
- Jianghua C, Wenqing X, Huiping W, et al. C4d as a significant predictor for humoral rejection in renal allografts. Clin Transplant. 2005:19:785–791
- Michaels PJ, Espejo ML, Kobashigawa J, et al. Humoral rejection in cardiac transplantation: Risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69
- Matheson PJ, Dittmer ID, Beaumont BW, et al. The macrophage is the predominant inflammatory cell in renal allograft intimal arteritis. Transplantation. 2005;79:1658–1662
- Tesch GH, Schwarting A, Kinoshita K, et al. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103:73–80
- Underhill DM, Bassetti M, Rudensky A, et al. Dynamic interactions of macrophages with T cells during antigen presentation. J Exp Med. 1999;190:1909–1914
- Langherhr JM, White DA, Hoffman RA, et al. Macrophages produce nitric oxide at allograft sites. Ann Surg. 1993;218:159–166
- Vandenbrodcke C, Caillat-Zucman S, Legendre C, et al. Differential in situ expression of cytokines in renal allograft rejection. Transplantation. 1991;51:602–609
- Pilmore HL, Yan Y, Eris JM, et al. Time course of upregulation of fibrogenic growth factors and cellular infiltration in a rodent model of chronic renal allograft rejection. Transpl Immunol. 2002;10:245–254
- Le Meur Y, Jose MD, Mu W, et al. Macrophage colony-stimulating factor expression and macrophage accumulation in renal allograft rejection. Transplantation. 2002;73:1318–1324
- Jose MD, Ikezumi Y, van Rooijen N, et al. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76:1015–1022
- Meehan SM, Siegel CT, Aronson AJ, et al. The relationship of untreated borderline infiltrates by the Banff criteria to acute rejection in renal allograft biopsies. J Am Soc Nephrol. 1999;10:1806–1814