Abstract
Background - Aim: In animal experiments, growth arrest-specific 6 (Gas6) protein plays a key role in the development of mesangial cell and glomerular hypertrophy in the early phase of diabetic nephropathy, and diabetic nephropathy is prevented by warfarin-induced inhibition of GAS6 protein. It was shown that GAS6 intron 8 c.834 + 7G > A polymorphism is protective against type 2 diabetes mellitus, and AA genotype is associated with higher blood levels of GAS6 protein. Our aim is to investigate whether this polymorphism is a risk factor for diabetic nephropathy in type 2 diabetes mellitus. Method: Eighty-seven patients with diabetic nephropathy were compared with 66 non-diabetic controls in terms of GAS6 intron 8 c.834 + 7G > A polymorphism. Patients with history of stroke, ischemic heart disease were excluded. Each patient was examined by the ophthalmologist to determine diabetic retinopathy. Results: Frequency of GG, GA and AA genotypes are similar in diabetic nephropathy and control groups according to GAS6 intron 8 c.834 + 7G > A polymorphism (p = 0.837). Rate of diabetic retinopathy was 54.02%. In the subgroup analysis, GA genotype was significantly more frequent than GG genotype in patients with diabetic retinopathy when compared to without diabetic retinopathy (p = 0.010). Conclusion: In our study, GAS6 intron 8 c.834 + 7G > A polymorphism was not associated with diabetic nephropathy in type 2 diabetes mellitus. However, heterozygous state of this polymorphism may be a risk factor for diabetic retinopathy in patients with diabetic nephropathy.
Introduction
Diabetic nephropathy is the most common cause of chronic kidney disease, and it accounts 45% of end-stage renal disease cases.Citation1 Diabetic nephropathy develops in about 20 to 40% of type 2 diabetes patients, and it is not known why it is not seen in all of the patients with diabetes mellitus type 2.Citation2 Many mechanisms may contribute to the emergence of diabetic nephropathy and its clinical course. It involves mainly the interaction between metabolic and hemodynamic changes caused by hyperglycemia and genetic predisposition.Citation2 Growth arrest-specific 6 (GAS6) was first discovered as a protein produced by growth arrested fibroblasts.Citation3 GAS6 is present at very low levels in the bloodstream (0.16–0.28 nm) in humans.Citation3 GAS6 protein is a member of the vitamin K-dependent coagulation proteins group, but in contrast to the other members, it plays a minor role in hemostasis GAS6 has a number of different functions concerning cellular survival, proliferation, migration and adhesion.Citation4 GAS6 exerts most of its effects over AXL, SKY and Mer2, which are members of AxL subfamily of tyrosine kinase receptors.Citation4 Animal studies showed that GAS6 protein plays a key role in the development of mesangial cell and glomerular hypertrophy in the early phase of diabetic nephropathy.Citation5,Citation6 When GAS6 is inhibited by warfarin, it was demonstrated that mesangial and glomerular hypertrophy is prevented, and hyperfiltration and albuminuria are reduced.Citation5,Citation6 Diabetic nephropathy was less severely observed in GAS6 knockout mice.Citation5,Citation6 A positive correlation was shown between albuminuria and serum levels of GAS6 in type 2 diabetic individuals.Citation1 Chromosomal localization of GAS6 gene is 13q34.Citation7 A single nucleotide polymorphism (c.834 + 7G > A) of this gene in intron 8 was demonstrated to be protective against stroke, diabetes and acute coronary syndrome.Citation4,Citation8,Citation9 We suggest that GAS6 intron 8 c.834 + 7G > A gene polymorphism might be a risk factor for diabetic nephropathy in type 2 diabetic individuals. In this study, we compared frequency of GAS6 intron 8 c.834 + 7G > A gene polymorphism between non-diabetic individuals and type 2 diabetic patients with diabetic nephropathy.
Methods
Study design and study population
This study was designed as a prospective, cross-sectional, case-control study. Records of diabetic patients followed-up with a diagnosis of type 2 diabetic nephropathy in Bezmialem Vakif University Medical Faculty Hospital, Internal Medicine Outpatient Clinic between December 2011 and December 2013 were screened. Patients meeting diabetic nephropathy diagnosis criteria according to the American National Kidney Foundation KDOQI Guidelines considering both clinical and routine laboratory findings were eligible for the study.Citation10 Patients who met all of the inclusion and exclusion criteria and gave informed consent were enrolled in this study. The control group consisted of non-diabetic patients accepted to give informed consent, admitted to internal medicine outpatient clinic for any reason subsequently, meeting the inclusion/exclusion criteria.
Inclusion criteria
Patients with type 2 diabetes mellitus and diabetic nephropathy or non-diabetic individuals between 18 and 80 years of age.
Exclusion criteria
Following individuals were excluded from the study: possibility of any nephropathy other than diabetic nephropathy associated with type 2 diabetes (nephrotic syndrome, rapidly decreasing glomerular filtration rate (GFR), rapidly increasing proteinuria, refractory hypertension, active urinary sediment, GFR decrease of more than 30% in 2 or 3 months following initiation of angiotensin converting enzyme inhibitor or angiotensin receptor blocker treatment), history of ischemic heart disease and/or stroke.
Criteria for diabetic nephropathy
Diagnostic criteria accepted for defining diabetic nephropathy in type 2 diabetic individuals is detection of urinary albumin/creatinine ratio of ≥300 mg/g creatinine in a first-morning void and detection of ≥300 mg/g creatinine in a first-morning void at the subsequent 2 measurements with 3 months interval together with no active urinary sediment, and provided that there is no other comorbidity that may cause proteinuria.
Criteria of being a non-diabetic subject
Persons who have not previously diagnosed with diabetes, provided that they are not on a diet, demonstrated that they have no diabetes mellitus following the oral glucose tolerance test performed with 75 g of oral glucose in the morning after overnight fasting were accepted as non-diabetic individuals.
Sampling and storing
Venous blood samples were obtained in the early morning after overnight fasting. Routine blood analyses were performed on the same day. Blood samples in EDTA containing tubes taken for GAS6 polymorphism was sent to the genetic laboratory immediately and DNA extraction was performed on the same day. A first morning void sample was taken for routine urinalysis and urine albumin/creatinine ratio, and it was analyzed on the same day.
Blood analysis
Complete blood cell (CBC) analysis was done with Sysmex XT 1800i apparatus (ROCHE-2011, Kobe, Japan). Biochemical assays were done on COBAS 8000 apparatus (ROCHE-2007, Tokyo, Japan) using COBAS-C system kits.
DNA extraction and genotype analysis
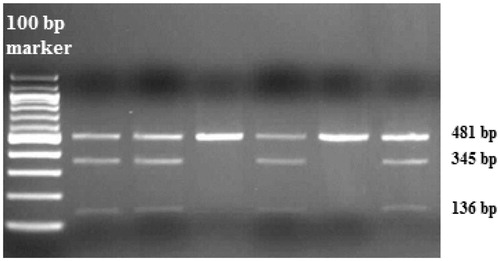
Blood samples were taken to the tubes containing EDTA and the genomic DNA was isolated using a DNA isolation kit (Invitrogen, Carlsbad, CA). All purified DNA samples were stored at +4 °C. Growth arrest-specific 6 (GAS6) gene polymorphism was analyzed with polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay.Citation11 For the genetic polymorphism rs8191974 (GAS6 c.834 + 7G > A in intron 8), 25 µl of total PCR mixtures containing 10.0 pmol of each primers, 1.0 mM dNTP (deoxynucleotide triphosphates), 25 mM MgCl2, 2.5U Taq DNA polymerase in the supplied reaction buffer (11615010, Invitrogen) and 100–200 ng of human genomic DNA were used. PCR was performed with the forward (5′-TTCCCTCAAGAAAGAGCCCG-3′) and reverse primers (5′-TCTCATCCCAAACCTCCACA-3′) with the initial denaturation at 94 °C for 5 min, following 33 cycles of 94 °C for 30 s, 62 °C for 30 s, 72 °C for 30 s and additionally the final step at 72 °C for 5 min. PCR products digested by AlwNI (R0514 S, NEB) were run on 2% agarose gel with ethidium bromide staining, then genotyped. Three genotypes for GAS6 gene polymorphism were defined by distinct banding patterns (); 481 bp for GG genotype; 481, 345 and 136 bp for GA genotype; and 345, 136 bp for AA genotype.
Ophthalmological examination
Each patient was examined for evaluation of diabetic retinopathy by the same ophthalmologist blinded to the study. The Airlie House classification modified by the Diabetic Retinopathy Study Research Group developed for the Early Treatment Diabetic Retinopathy Study was used for grading retinopathy.Citation12,Citation13
Statistics
Numerical variables are presented as means with standard deviations, and nominal variables in ratios. Nominal and numerical independent variables were compared between the diabetic nephropathy group and the control group. The chi-square test was used to compare nominal independent variables. One sample Kolmogorov-Smirnov test was done to see if the continuous independent variables were normally distributed. Normally distributed independent continuous variables were compared with Student’s t-test; whereas non-normally distributed independent continuous variables were compared with the Mann–Whitney U-test. A two-tailed p-value of <0.05 was considered to be statistically significant.
Ethics
The study was approved by the Ethics Committee of the Bezmialem Vakif University and written informed consents were obtained from all the patients. The study was conducted in accordance with the Helsinki Declaration of 2009.
Results
There were 153 individuals in the study. Nephropathy and control groups consisted of 87 and 66 individuals, respectively. In the nephropathy group there were 56 female and 31 male subjects, and in the control group there were 41 female and 25 male subjects (p = 0.775). In the study group, mean age was 57.9 ± 10.2 years (19–80 years) (p > 0.05, ). Mean BMI was 31.6 ± 5.8 kg/m2 (16.9–44.9 kg/m2) in our study (p > 0.05, ).
Table 1. Demographics and routine laboratory tests in two groups.
Hypertension was significantly more frequent in the diabetic nephropathy group, also arterial blood pressure was significantly higher ( and ). According to GAS6 intron 8 c.834 + 7G > A gene polymorphism analysis, there is no significant difference in terms of hypertension frequency among GG, GA and AA genotypes in both diabetic nephropathy group and control group (p = 0.365 and p = 0.124, respectively). Also in diabetic nephropathy group albuminuria was similar among GG, GA and AA genotypes (p = 0.313).
Table 2. Frequency of hypertension and antihypertensive drugs, which patients used regularly between two groups.
Frequency of GG, GA and AA genotypes are similar in diabetic nephropathy and control groups in the analysis of GAS6 intron 8 c.834 + 7G > A polymorphism ().
Table 3. Frequency of GAS6 intron 8 c.834 + 7G > A gene polymorphism in diabetic nephropathy and control groups.
The frequency of GA or AA genotype versus GG genotype in diabetic nephropathy and control groups according to GAS6 intron 8 c.834 + 7G > A polymorphism analysis were compared by the chi-square test, and found similar (). So the frequency allele A was similar in diabetic nephropathy and control groups.
Diabetic retinopathy was present in 47 out of 87 patients with diabetic nephropathy (54.02%). Fourteen of the patients with diabetic retinopathy have mild non-proliferative retinopathy, 12 have moderate non-proliferative retinopathy, 12 have severe non-proliferative retinopathy and nine have proliferative retinopathy. The frequency of GAS6 intron 8 c.834 + 7G > A polymorphism was compared in diabetic nephropathy patients with and without diabetic retinopathy using the chi-square test. AA and GG genotypes were found with similar frequency in the two groups (p = 0.745). However, GA genotype was significantly more frequent than GG genotype in diabetic nephropathy patients with diabetic retinopathy (). GA genotype represents a significantly increased risk ratio for diabetic retinopathy in patients with diabetic nephropathy (OR: 1.68, 95% Confidence interval: 1.12 to 2.50, p = 0.010). AA genotype was observed less frequently in diabetic nephropathy patients with diabetic retinopathy compared to the diabetic nephropathy patients having no diabetic retinopathy, but without statistical significance (, p = 0.215).
Table 4. Frequency of GAS6 intron 8 c.734 + 7G > A gene polymorphism in diabetic nephropathy patients with diabetic retinopathy and without retinopathy.
Discussion
Revealing the pathogenesis of diabetic nephropathy has paramount importance since diabetic nephropathy is the most common cause of end-stage renal failure. Expansion of mesangium, hypertrophy of glomerulus, increased glomerular filtration rate and albuminuria are observed in early phase of diabetic nephropathy.Citation5,Citation6 If not intervened, consequences of these changes are glomerulosclerosis and reduced glomerular filtration.Citation5,Citation6 Since diabetic nephropathy is reversible in the early phase it is particularly important to reveal molecular mechanisms of glomerular mesangial expansion and hypertrophy.Citation5,Citation6 The relationship of GAS6 with diabetic nephropathy was shown in animal studies. In streptozotocin-induced diabetic rat’s glomerular hypertrophy, increased GFR and albuminuria were observed 12 weeks after STZ injection.Citation5,Citation6 The expression of GAS6 and AXL receptors were found elevated in the glomeruli of these rats.Citation5,Citation6 Since warfarin inhibits the synthesis of GAS6 protein, low-dose warfarin administration was demonstrated to inhibit albuminuria, GFR increase and glomerular hypertrophy in streptozotocin-induced diabetic rats.Citation5,Citation6 When diabetes was induced in GAS6 knockout and wild type mice, it was seen that mesangial expansion and glomerular hypertrophy were mild in GAS6 -/- mice.Citation5,Citation6 It was demonstrated that GAS6 added to cell culture medium in concentrations lower than the required concentration to exhibit mitogenic activity causes mesangial cell hypertrophy.Citation5,Citation6 These studies suggest that GAS6-Axl pathway may have an important role in the early phase of diabetic nephropathy.Citation5,Citation6
Besides the animal experiments and cell culture studies, our previous study has shown that in type 2 diabetic patients with micro- and macroalbuminuria, GAS6 concentration was higher than the control group, and positive correlation exists between albuminuria and plasma GAS6 levels.Citation1
In a recent study comparing GAS6 intron 8 c.834 + 7G > A polymorphism in subjects with type 2 diabetes, impaired glucose tolerance and controls, it was shown that AA genotype is protective against type 2 diabetes, and for individuals with AA genotype, plasma GAS6 protein levels are higher compared to individuals with GG genotype.Citation8 This genotype was also found to be protective from type 2 diabetes as well as acute coronary syndrome and stroke.Citation4,Citation8,Citation9 It was shown that fasting glucose, TNF-alpha, interleukin-6 and vascular cell adhesion molecule-1 levels decrease when GAS6 protein plasma concentration increases. That means GAS6 is related to diabetes, inflammation and endothelial function.Citation14 For this reason, AA genotype associated with higher GAS6 protein levels may protect against diabetes mellitus type 2, acute coronary syndrome and stroke. As in animal experiments it was shown that GAS6 protein is responsible for mesangial expansion and glomerular hypertrophy during the early phase of diabetic nephropathy.Citation5,Citation6 In this case, higher GAS6 protein levels may not be protective against diabetic nephropathy but contrarily may facilitate development of it. We suggested that AA or GA genotypes of GAS6 intron 8 c.834 + 7G > A polymorphism associated with higher plasma GAS6 protein levels might be associated with higher incidence of diabetic nephropathy. However, in this study, AA or GA genotype is not more frequent in diabetic nephropathy patients compared to the control group. Hence, we determined that allele A is not associated with diabetic nephropathy.
Although diabetic nephropathy seems in direct proportion with poor glycemic control or duration of diabetic state, this is not applicable for all patients with diabetic nephropathy.Citation2 Nevertheless, diabetic nephropathy is only present in about 30% of diabetic patients.Citation2 It is obvious that a genetic predisposition should be present for the emergence of diabetic nephropathy.Citation2 In a study conducted on Pima Indians, among those families having diabetes for two successive generations, if proteinuria is not present in both parents, the probability of developing overt proteinuria in siblings is 14%; however, if proteinuria is present in one or both of the parents, then probability is 23 and 46%, respectively.Citation2 In Pima Indians, 7q21.3, 10p15.3, 14q23.1 and 18q22.3 chromosome gene loci were identified leading to a susceptibility to diabetic nephropathy.Citation2 It has been suggested that genetic variations in ACE gene are associated with diabetic nephropathy.Citation2 In type 2 diabetic patients, it was demonstrated that DD polymorphism of ACE gene is associated with the development of diabetic nephropathy, severe proteinuria, progressive renal failure and mortality during dialysis.Citation2 However, in other studies investigating the association between diabetic nephropathy and ACE gene polymorphism, conflicting results were obtained.Citation2
The subgroup analysis we performed revealed that GA genotype in the GAS6 intron 8 c.834 + 7G > A polymorphism is significantly more frequent in diabetic nephropathy patients with diabetic retinopathy compared to patients without retinopathy. As a result, GA genotype seems to be a risk factor for retinopathy in patients with diabetic nephropathy. AA genotype was tended to be present less frequently in diabetic nephropathy and retinopathy patients. If we have larger diabetic nephropathy patient population, it might be revealed that AA genotype is protective against retinopathy in diabetic nephropathy contrary to GA genotype. Diabetic retinopathy is the leading cause of blindness in the USA for adults aged 20–74.Citation15 The risk of the development of diabetic retinopathy is related to poor glycemic and blood pressure control and the duration of diabetes similar to nephropathy.Citation15 Similarly diabetic retinopathy does not develop in all patients with these risk factors.Citation15 It is obvious that genetic predisposition is necessary for the development of diabetic retinopathy. Heritability was reported as 27% for diabetic retinopathy, and 52% for proliferative diabetic retinopathy and advanced diabetic proliferative retinopathy.Citation15 Despite these findings, to our knowledge, linkage analyses, candidate gene association studies and genome wide association studies could not demonstrate a widely reproducible diabetic retinopathy locus. Whether GAS6 gene polymorphism is a risk factor for the development of diabetic retinopathy is yet to be studied. In our study, we demonstrated that among diabetic nephropathy patients GAS6 intron 8 c.834 + 7G > A polymorphism is associated with diabetic retinopathy. Whether GAS6 intron 8 c.834 + 7G > A gene polymorphism is a risk factor for diabetic retinopathy independent from diabetic nephropathy should be investigated in broader patient populations.
In conclusion, there is no correlation between GAS6 intron 8 c.834 + 7G > A gene polymorphism and diabetic nephropathy in type 2 diabetic individuals. Among diabetic nephropathy patients GAS6 intron 8 c.834 + 7G > A polymorphism is associated with diabetic retinopathy. It is suggested that genetic basis of type 2 diabetes involves interaction between many genes.Citation16 Like this, genetic basis of diabetic nephropathy may not be explained with a single gene.
Declaration of interest
All authors declare there is no conflict of interest.
References
- Erek-Toprak A, Bingol-Ozakpinar O, Karaca Z, et al. Association of plasma growth arrest-specific protein 6 (Gas6) concentrations with albuminuria in patients with type 2 diabetes. Ren Fail. 2014;36:737–742
- Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452
- Ekman C, Jonsen A, Sturfelt G, Bengtsson AA, Dahlback B. Plasma concentrations of Gas6 and sAxl correlate with disease activity in systemic lupus erythematosus. Rheumatology. 2011;50:1064–1069
- Munoz X, Obach V, Hurtado B, de Frutos PG, Chamorro A, Sala N. Association of specific haplotypes of GAS6 gene with stroke. Thromb Hemost. 2007;98:406–412
- Arai H, Nagai K, Doi T. Role of growth arrest-specific gene 6 in diabetic nephropathy. Vitam Horm. 2008;78:375–392
- Yanagita M. Gas6, warfarin, and kidney diseases. Clin Exp Nephrol. 2004;8(4):304–309
- Saccone S, Marcandalli P, Gostissa M, Schneider C, Della Valle G. Assignment of the human GAS6 gene to chromosome 13q34 by fluorescence in situ hybridization. Genomics. 1995;30:129–131
- Lee CH, Chu NF, Shieh YS, Hung YJ. The growth arrest-specific 6 (Gas6) gene polymorphism c.834 + 7G > A is associated with type 2 diabetes. Diabetes Res Clin Pract. 2012;95:201–206
- Jiang L, Liu CY, Yang QF, Wang P, Zhang W. Plasma level of growth arrest-specific 6 (GAS6) protein and genetic variations in the GAS6 gene in patients with acute coronary syndrome. Am J Clin Pathol. 2009;131:738–743
- KDOQI. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154
- Wu CS, Hu CY, Chan CJ, Chang SK, Hsu PN. Genetic polymorphism of the growth arrest-specific 6 gene is associated with cutaneous vasculitis in Taiwanese patients with systemic lupus erythematosus. Clin Rheumatol. 2012;31:1443–1448
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs – An extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98(5 Suppl):786–806
- Wu L, Fernandez-Loaiza P, Sauma J, Hernandez-Bogantes E, Masis M. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;15:290–294
- Hung YJ, Lee CH, Chu NF, Shieh YS. Plasma protein growth arrest-specific 6 levels are associated with altered glucose tolerance, inflammation, and endothelial dysfunction. Diabetes Care. 2010;33:1840–1844
- Cho H, Sobrin L. Genetics of diabetic retinopathy. Curr Diabetes Rep. 2014;14(8):515
- So WY, Ng MC, Lee SC, Sanke T, Lee HK, Chan JC. Genetics of type 2 diabetes mellitus. Hong Kong Med J. 2000;6:69–76