Abstract
Inflammation is a key part in the etiology and progression of idiopathic nephrotic syndrome (INS), we hypothesize that removing pro-inflammatory cytokines with intermittent high-volume hemofiltration (IHVHF) could improve the outcome in INS patients. The purpose of the current study is to examine whether IHVHF promotes remission in steroid-resistant INS. Fifty-one steroid-resistant INS patients were followed up on an open-label basis with prospective evaluations. Thirty-five patients received mycophenolate mofetil (SRD group) and 16 patients received drugs and IHVHF due to volume overload despite of diuretics (SRDF group). The rate of complete remission (CR) was analyzed. We also recruited 30 healthy individuals and 36 steroid-sensitive (SS) INS patients as controls to investigate the correlation of interleukin (IL)-8, IL-10, IL-6 and IL-17 with INS activity. Compared with the patients in the SRD group, the 6-month CR rate was higher (44% vs. 9%, p < 0.001) and time to first CR was significantly shorter (7.3 ± 3.6 vs. 11.1 ± 5.3 months, p = 0.02) in the SRDF group. Serum IL-8 was highest in the SRDF group and reduced by IHVHF clearance. Serum IL-8 was lower during remission than at onset or recurrence of INS, whereas no significant difference was seen in the other cytokines. Receiver operating characteristic curve analysis demonstrated that serum IL-8 predicted steroid sensitivity with moderate accuracy (area under the curve = 0.79, 95% CI: 0.69–0.87). IHVHF promotes remission in patients with steroid-resistant INS and it may be partly due to serum IL-8 clearance.
Introduction
In patients with idiopathic nephrotic syndrome (INS), massive proteinuria is an independent risk factor for progression to end-stage renal disease.Citation1 Glucocorticoids have been used as first-line drugs for the treatment of INS for several decades.Citation2 However, individual sensitivity to glucocorticoids differs, and 50–80% of adult patients are steroid resistant.Citation3,Citation4 The complete remission (CR) rate of steroid-resistant nephrotic syndrome is only 27.2–48.5%, despite combined treatment with immunosuppressants.Citation5–7 We have used intermittent, high-volume, hemofiltration (IHVHF) in INS patients who had steroid and immunosuppressant resistance along with severe volume overload and heart failure unresponsive to diuretics. Interestingly, we found that IHVHF not only effectively corrected volume overload, but also significantly reduced proteinuria, even though their immunosuppressive medications were the same as those before IHVHF. Therefore, in this prospective study, we have, for the first time, investigated whether IHVHF reduces proteinuria and improves CR in INS patients.
Although studies have found a beneficial effect of plasma exchange and lipoprotein replacement on steroid resistance,Citation8,Citation9 the underlying mechanism remains unknown. Shalhoub first hypothesized that certain circulating factors increase the protein permeability of the glomerular filtration barrier and researchers have further postulated that the major “circulating factors” responsible for steroid resistance are cytokines derived from dysfunctional T cells.Citation10–12 As IHVHF can clear low-to-middle-molecular-weight components from the blood, we speculated that the improvement in the prognosis of INS patients was attributable to the clearance of cytokines responsible for steroid resistance from the peripheral circulation. Since T-helper (Th)2 cell dominance is associated with steroid resistance in INS,Citation13 we examined serum level of representative pro-inflammatory cytokines, including interleukin (IL)-8, and IL-6, as well as the anti-inflammatory cytokine IL-10. Considering that IL-17 has also been implicated in steroid resistance,Citation14 IL-17 was also examined. Therefore, in our study, we also measured the cytokines in INS patients who received steroid treatment at disease onset, remission and recurrence, in order to explore the correlation of cytokines with IHVHF clearance and INS activity.
Methods
Study participants
This single-center, prospective, open-label study involved all patients with INS admitted to the Division of Nephrology, the First Affiliated Hospital of Dalian Medical University since January 2009. The inclusion criteria were (a) age, 18–70 years, (b) treatment with glucocorticoids for 8 weeks, urine protein ≥3.5 g/24 h and serum albumin <30 g/L, (c) no history of hypertension or effective control of blood pressure to ≤130/80 mm Hg, (d) treatment with ARBs or ACEIs for more than 8 weeks, (e) serum creatinine, ≤200 μmol/L and (f) routine renal biopsy. The exclusion criteria were (a) various secondary nephrotic syndromes, and (b) body mass index, ≤18 or ≥25 kg/m2. In addition, we recruited healthy individuals as healthy controls and steroid-sensitive INS patients as disease controls. The age, gender and race of the controls were matched with those of the SR patients. The study was approved by the ethics committee of the First Affiliated Hospital of Dalian Medical University at 2008. All subjects provided their written informed consent and the study got registration in Chinese Clinical Trial Registry (chiCTR-ONC-12002809).
Treatments
Patients aged <60 years received 500 mg/d methylprednisolone intravenously for 3 d, followed by 40 mg/d oral prednisone. Patients aged ≥60 years were administered 250 mg/d methylprednisolone intravenously for 3 d, followed by 30 mg/d oral prednisone. After 8 weeks of this therapy, the prednisone dose was tapered using 2.5 mg decrements every 2 weeks until it reached 20 mg/d. If patients entered a CR, the prednisone reduction was continued until the dose reached 10 mg, and this dose was maintained for 8 weeks until drug withdrawal. If CR was not achieved, the prednisone dose was maintained at 20 mg for 8 weeks and then tapered until drug withdrawal.
The patients were divided into the following groups according to the further treatments administered after initial 8-week-long prednisone treatment. The SRD group included patients who received 1.5 g/d mycophenolate mofetil (MMF) q12h for 12 months. The SRDF group included 16 patients who presented severe edema during treatment, heart failure in 6 cases and oliguria/ascites in 10 cases. Furosemide infusion was applied after albumin administration and the daily dosage of furosemide was 162 (125–207) mg. In 8 week, their body weight increased 15.7 (10.2–18.4) kg and daily growth was more than 2 kg. Plasma B-type natriuretic peptide (BNP) detected by electrochemiluminescence was 2876 (1547–4214) pg/mL. Therefore, these patients received glucocorticoid combined MMF and IHVHF treatment. IHVHF was provided using the following parameters: an Aquarius system (Baxter), 8 h sessions, 1.2 m2 polysulfone membrane filter, a fluid displacement velocity of 4 l/h in the pre-dilution mode, a blood flow rate of 200 mL/min and anti-coagulation with low-molecular-weight heparin.
Outcome measures
CR was defined as urine protein ≤0.3 g/24 h, serum albumin ≥35 g/L and normal serum creatinine. In patients who achieved CR, recurrence was defined as a 30% increase in the 24 h urine protein level that could not be relieved within 2 weeks (after eliminating fatigue and infection). For each patient, the data at 8 week taking prednisone was set as baseline. All patients were followed up once a month after the treatments and once every 3 months after CR. At each follow-up, serum creatinine was measured with an enzymatic assay and renal function was reflected using estimated glomerular filtration rate (eGFR), determined using the Modification of Diet in Renal Disease equation modified for the Chinese population.Citation15 The laboratory indexes studied also included serum albumin, 24 h urine protein, and blood lipid and fibrinogen levels.
Enzyme-linked immunosorbent assays for cytokines
All patients underwent venous blood collection in the morning within 1 week of onset, after CR and after the first recurrence. Among patients who underwent IHVHF, pre- and post-hemofiltration venous blood was collected at initiation, 2, 4, 6 and 8 h, and the ultrafiltrate was collected simultaneously for sieving coefficient (SC) calculation: SC = 2 × cytokine level in ultrafiltrate/(cytokine level in pre-hemofilter blood + cytokine level in post-hemofilter blood). Thus, cytokine clearance (Cl) was calculated as follows: Cl = SC × ultrafiltrate flow rate.Citation16 All blood samples were centrifuged at 2000 g for 10 min within 2 h of collection, and sera were stored at −70 °C. The cytokine levels in the collected sera and ultrafiltrate at each time point were measured using enzyme-linked immunosorbent assay (R&D System, Minneapolis, MN).
Statistical analysis
SPSS version 18.0 was used for statistical analyses (Chicago, IL). The results were expressed as the mean and standard deviation (SD) or median and range, according to the normality of each variable, with a 5% significance level. For normally distributed continuous variables, we used an independent t-test or one-way analysis of variance (ANOVA). For repeated-measures data, we used the repeated-measures ANOVA. In the case of non-normally distributed data, the Mann–Whitney U test was used for comparisons between two groups, and the Kruskal–Wallis H test was used for comparisons between multiple groups. Categorical variables were expressed as proportions and compared using the chi-square test. Sensitivity and specificity were calculated, and the accuracy of the prediction of the effects of steroid therapy was assessed using ROC curves. The cumulative CR was analyzed using Kaplan–Meier curves and the log-rank test. The level of significance was set at p < 0.05.
Results
Study participants
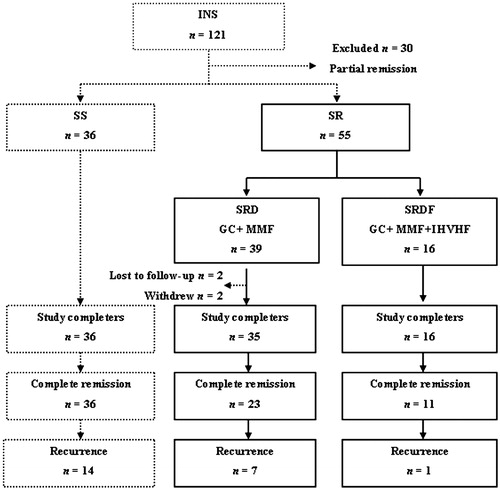
This study included 121 INS patients, thirty partial remission patients were excluded after initial 8 weeks of steroids. Thirty-six patients were defined as the steroid-sensitive (SS) group. All the SS patients achieved CR, but 14 of them patients developed recurrence. Fifty-five patients were defined as the steroid-resistant (SR) group and they received further treatment. Among these patients, 39 were included in the steroid resistance and drugs (SRD) group. Of these, two patients were excluded from the final analysis due to severe infection, and two patients were lost to follow-up; the remaining 35 patients completed follow-up. CR occurred in 23 of the 35 SRD-group patients, but seven of them developed recurrence. Sixteen patients underwent IHVHF to form the steroid resistance with drugs and IHVHF (SRDF) group, and all of them completed follow-up. Of the 11 SRDF-group patients who achieved CR, one patient developed recurrence ().
Figure 1. Flowchart of patient selection. INS, idiopathic nephrotic syndrome; SS, steroid-sensitive group; SR, steroid-resistant group; SRD, steroid resistance with drugs group; SRDF, steroid resistance with drugs and hemofiltration group; GC, glucocorticoid; MMF, mycophenolate mofetil; IHVHF, intermittent, high-volume hemofiltration.
Baseline clinical characteristics
The baseline clinical data of the patients is shown in . No difference was noticed in age and gender between the two arms. Subjects in the SRDF group had higher serum creatinine (p < 0.001) and lower eGFR (p < 0.001) at the baseline. Urinary output in the SRDF group was lower than in the SRD group (p < 0.001). Sever edema was apparent in the SRDF group, and not in the SRD group. Plasma BNP level in the SRDF group was higher than in the SRD group (p < 0.001). The other laboratory indexes including 24 h-proteinuria and pathological types of the underlying renal diseases did not differ significantly between the two arms.
Table 1. Baseline clinical characteristics of the SRD- and SRDF-group patients.
Outcome measures
The median follow-up duration in the SRD and SRDF groups was 29 months (range, 19–31 months) and 27 months (range, 11–33 months), respectively. Severe edema was corrected by IHVHF in all 16 cases of the SRDF group. The total duration of IHVHF was 28 (24–40) h, and the total ultrafiltration volume was 14.5 (12–20.8) kg. For the patients in the SRDF group after IHVHF treatment, body weight declined by 16.4 (12.7–22.8) kg, urinary output increased to 1.8 (1.2–2.2) l/d, and plasma BNP decreased to 156 (89–345) pg/mL. The cumulative dosage of oral glucocorticoids was 1270 (1270–1430) mg and 760 (760–1270) mg in the SRD and SRDF groups, respectively (p = 0.032).
The log-rank test showed that at the 36-month follow-up, the cumulative CR rate did not differ between the SRD and SRDF groups (p = 0.35), but the curves had shifted to the left in the SRDF group (). The time to first CR was obviously shorter in the SRDF group than in the SRD group (p = 0.021, ). In addition, the CR rate at the 6-month follow-up was significantly higher in the SRDF group than in the SRD group (p < 0.001, ), but there was no difference between the two groups after 12 months.
Figure 2. Renal function and proteinuria remission during follow-up in patients in the steroid resistance with drugs (SRD) and steroid resistance with drugs and hemofiltration (SRDF) groups. (A) Cumulative complete remission. (B) Time to complete remission. (C) Complete remission at specific time points. (D) Serum creatinine. (E) Estimated glomerular filtration rate (eGFR). (F) Serum albumin. (G) Levels of 24 h urine protein excretion. (H) Proteinuria reduction in patients entering a complete remission. p Values are for SRD vs. SRDF, *p < 0.01.
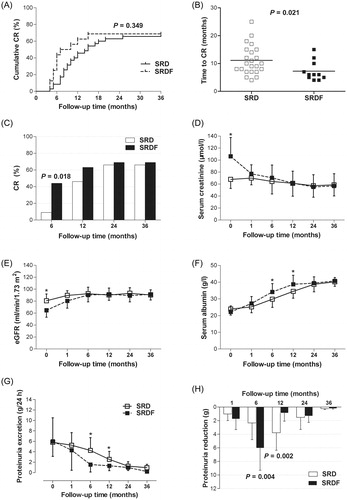
In the SRDF group, serum creatinine at the 1-month follow-up after IHVHF decreased from a baseline of 106.2 ± 31.4 μmol/L to 77.1 ± 15.1 μmol/L (p < 0.001); similarly, eGFR increased from a baseline of 64.7 ± 11.5 mL/min/1.73m2 to 80.4 ± 12.2 mL/min/1.73m2 (p < 0.001). At subsequent follow-up visits, serum creatinine and eGFR were maintained at normal levels in both the SRDF and SRD groups ().
At the 6 - and 12-month follow-up examinations, the urine protein level was lower (p < 0.001) and the serum albumin level was higher (p < 0.001) in the SRDF group than in the SRD group. However, no significant differences were observed at the 24 - and 36-month follow-up examinations (). Specifically, the maximum reduction in proteinuria in the SRDF-group patients who achieved CR occurred at the 6-month follow-up (p = 0.004), whereas in the SRD-group patients, this occurred at the 12-month follow-up (p = 0.002; ).
Effect of IHVHF on cytokine levels
Because IHVHF could clear cytokines, we further assessed the effect of IHVHF on cytokine levels. After IHVHF treatment, the serum IL-8 level significantly decreased from 287.4 ± 118.2 pg/mL to 221.5 ± 120.0 pg/mL (p < 0.001), and the serum IL-6 level significantly decreased from 163.8 ± 100.4 pg/mL to 123.9 ± 85.89 pg/mL (p < 0.001). The serum IL-10 level slightly increased from 157.5 ± 93.7 pg/mL to 186.0 ± 84.1 pg/mL (p = 0.04), while the serum IL-17 level remained unchanged (p = 0.12; ). The peak IL-8 clearance was at 4 h of treatment, and the peak IL-10 clearance was at 2 h. IL-6 and IL-17 clearance were stable throughout IHVHF ().
Figure 3. Influence of intermittent, high-volume hemofiltration (IHVHF) on serum cytokine levels in the patients in the steroid resistance with drugs and hemofiltration (SRDF) group. IL, interleukin.
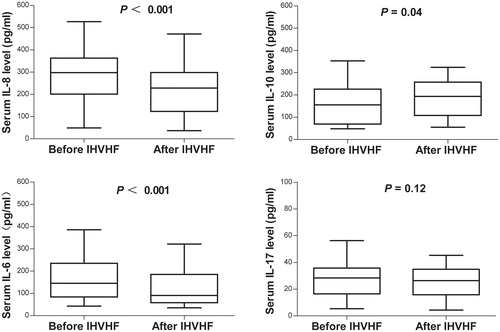
Table 2. Cytokine clearance (Cl) with intermittent, high-volume hemofiltration (IHVHF).
Serum IL-8 level as a predictor of disease activity and steroid sensitivity
Prior to the initiation of therapy, the serum IL-8, IL-10 and IL-6 levels were higher in INS patients than in healthy participants. However, only the serum IL-8 level was higher in the SRD and SRDF groups than in the SS group, with the highest levels being observed in the SRDF group. The serum IL-10 and IL-6 levels did not differ among the SS, SRD and SRDF groups, and the serum IL-17 level was similar in all groups ().
Table 3. Serum cytokine levels in controls and patients with idiopathic nephrotic syndrome (INS) at disease onset.
We next investigated the correlation of cytokines with recurrence of INS. We found that the serum IL-8 level was lower during the remission period than at the time of disease onset and recurrence (p < 0.001); however, the levels of the other cytokines did not vary at these three time points ().
Figure 4. Relationship of serum interleukin (IL) levels with disease activity and sensitivity to glucocorticoids in patients with idiopathic nephrotic syndrome (INS). (A) Serum cytokine levels and INS activity. (B) Area under the receiver operating characteristic curve (AUC) suggests that serum IL-8 level at INS onset is a predictor of sensitivity to glucocorticoids.
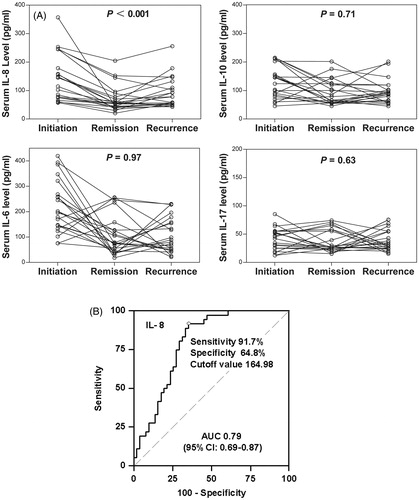
The accuracy of serum IL-8 at onset in the prediction of steroid sensitivity was assessed using receiver operating characteristic (ROC) curve analysis (). The ROC curve analysis of serum IL-8 at onset alone revealed an area under the curve of 0.79 (95% CI: 0.69–0.87). As a predictor of steroid sensitivity, serum IL-8 at onset achieved a sensitivity of 91.7% and a specificity of 64.8% at an optimal cutoff value of 164.98 pg/mL ().
Discussion
IHVHF, which can clear excess cytokines to reduce the cascade effect of inflammatory molecules , has been effectively used to treat severe infections such as sepsis,Citation17,Citation18 but has not yet been reported to treat INS. In the present study, IHVHF could rapidly and effectively improve renal function. We also found that 44% of the patients in the SRDF group and only 9% of the patients in the SRD group had achieved CR at 6 months of follow-up. As the drugs administered were the same in both groups, this finding strongly suggested that IHVHF plays a direct role in reducing proteinuria. Owing to the rapid reduction in urine proteins in the SRDF group, the rate of the steroid reduction was also faster, it can shorten the course of treatment and avoid steroid-related side effects. Therefore, IHVHF enables rapid steroid-resistant nephrotic syndrome remission and the beneficial effects of IHVHF are not merely due to the clearance of toxins and water, but also involves repair of the glomerular pathological injury. Studies have showed that blood purification therapy can improve the immune status of patients with sepsis.Citation19 This effect may be attributable to the improvement in immune status after cytokine clearance. Our study also indicated that IHVHF has a similar potential effect on patients with glomerular disease associated with massive proteinuria, though further experiments are needed to verify this result.
IL-8 and IL-6, the proinflammatory factors secreted by Th2 cells, exacerbate local renal and systemic inflammation.Citation20,Citation21 IL-10, an anti-inflammatory factor secreted by Th2 cells, can inhibit many chemokines like IL-8 that participate in inflammation and has a certain immune-regulation effect. The present study found that the serum IL-8 and IL-6 levels dropped after IHVHF therapy, while the serum IL-10 level slightly increased. The peak IL-10 clearance was earlier than the peak IL-8 clearance. Numerous inflammatory factors may be produced during the early phase of inflammatory reaction; however, an immune paralysis may subsequently result because of an increase in anti-inflammatory factors.Citation22 High-volume hemofiltration promotes the recovery of immune homeostasis in patients with sepsis and in animal models,Citation23,Citation24 via improvement in T-cell function and recovery of Thl/Th2 balance. Thus, although both pro- and anti-inflammatory factors were initially cleared during IHVHF, Th2 cell function was ultimately improved partly due to the later clearance of the proinflammatory factor.
From the serum cytokine levels before and after therapy, we deduced that IHVHF can partially influence the improvement in steroid efficacy by clearing away serum IL-8, IL-6 and IL-10. We therefore compared serum cytokine levels between INS patients and healthy controls. The results showed that IL-8, IL-6 and IL-10 levels significantly differed between the groups; however, only the IL-8 level differed between the SRD and SRDF groups. Serum IL-6 and IL-10 levels were not related to either steroid effect or disease activity. IL-8 is also secreted by podocytes,Citation25 and can lead to proteinuria by influencing sulfide metabolism in the glomerular basement membrane.Citation26 Therefore, the higher serum IL-8 levels in SRDF group might be due to the aggravation of renal injury in these patients. Moreover, the serum IL-8 level could reflect INS development and remission; further investigation demonstrated that it could predict steroid sensitivity with moderate accuracy. This suggested that although IHVHF produces less impact on the steroid sensitivity although it can clear away IL-6 and IL-10. Increased Th17 cell counts have been found in INS patients with impaired renal function.Citation27 However, serum IL-17, a proinflammatory factor secreted by TH17 cells, ,did not differ in our study.
There are two major limitations to this study. First, intervention was adopted in accordance with the severity of the patients’ condition, rather than in a random manner; however, in the SRDF group, the baseline renal function was worse than that in the SRD group, and it obviously improved after IHVHF treatment. Therefore, IHVHF showed distinct therapeutic value in intractable INS, this is, to our knowledge, the first study to focus on the improvement of steroid sensitivity by IHVHF. Second, because of the limited sample size, we did not attempt to compare cytokine levels between patients with different pathological types. Studies have shown that the traditional histopathology of INS determines the response to steroid treatment to a certain extent. Podocytopathy, a spectrum of proteinuric glomerular diseases results from podocyte abnormalities, has been presented as an expanded concept for reassessment of the primary nephrotic diseases in recent years.Citation28,Citation29 The pathological types in this study have been involved in podocytopathies, and it may offer a framework that integrates the morphology and pathogenesis in the diagnosis of the podocytopathies to investigate the possible underlying disorders.
In INS patients, IHVHF can correct volume overload that is unresponsive to diuretics and improve steroid sensitivity partly due to the serum clearance of the proinflammatory factor IL-8. Therefore, IHVHF was an effective alternative for patients with intractable INS.
Declaration of interest
This study was supported by grants from the General Program of National Natural Science Foundation of China (No. 81200522).
References
- Erickson FK, Lea J, McClellan WM. Interaction between GFR and risk factors for morbidity and mortality in African Americans with CKD. Clin J Am Soc Nephrol. 2013;8(1):75–81
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – New mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723
- Ponticelli C, Zucchelli P, Passerini P, Cesana B. Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. N Engl J Med. 1992;327(9):599–603
- Rydel JJ, Korbet SM, Borok RZ, Schwartz MM. Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis. 1995;25(4):534–542
- Chen W, Liu Q, Liao Y, et al. Outcomes of tacrolimus therapy in adults with refractory membranous nephrotic syndrome: a prospective, multicenter clinical trial. Am J Med Sci. 2013;345(2):81–87
- Gulati A, Sinha A, Jordan SC, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: Multicentric report. Clin J Am Soc Nephrol. 2010;5(12):2207–2212
- Aizawa-Yashiro T, Tsuruga K, Watanabe S, Oki E, Ito E, Tanaka H. Novel multidrug therapy for children with cyclosporine-resistant or -intolerant nephrotic syndrome. Pediatr Nephrol. 2011;26(8):1255–1261
- Feld SM, Figueroa P, Savin V. Plasmapheresis in the treatment of steroid-resistant focal segmental glomerulosclerosis in native kidneys. Am J Kidney Dis. 1998;32(2):230–237
- Sato Y, Tsunoda S, Nozue T, Pan Q, Wakasugi H, Yoshimura A. Low-density lipoprotein apheresis therapy for steroid- and cyclosporine-resistant idiopathic membranous nephropathy. Intern Med. 2012;51(18):2597–2602
- Shalhoub RJ. Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet. 1974;2:556–560
- Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883
- McCarthy ET, Sharma M, Savin VJ. Circulating permaebility factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2011;26(8):1255–1261
- Kanai T, Shiraishi H, Yamagata T, et al. Th2 cells predominate in idiopathic steroid-sensitive nephrotic syndrome. Clin Exp Nephrol. 2010;14:578–583
- Fouser LA, Wright JF, Dunussi-Joannopoulos K, Collins M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev. 2008;226:87–102
- Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944
- Morgera S, Slowinski T, Melzer C, et al. Renal replacement therapy with high-cutoff hemofilters: Impact of convection and diffusion on cytokine clearances and protein status. Am J Kidney Dis. 2004;43:444–453
- Ratanarat R, Brendolan A, Ricci Z, et al. Pulse high-volume hemofiltration in critically ill patients: a new approach for patients with septic shock. Semin Dial. 2006;19(1):69–74
- Peng Z, Pai P, Han-Min W, et al. Evaluation of the effects of pulse high-volume hemofiltration in patients with severe sepsis: A preliminary study. Int J Artif Organs. 2010;33(8):505–511
- Gong D, Zhang P, Ji D, et al. Improvement of immune dysfunction in patients with severe acute pancreatitis by high-volume hemofiltration: A preliminary report. Int J Artif Organs. 2010; 33(1):22–29
- Souto MF, Teixeira AL, Russo RC, et al. Immune mediators in idiopathic nephrotic syndrome: Evidence for a relation between interleukin 8 and proteinuria. Pediatr Res. 2008;64(6):637–642
- Ghani RA, Zainudin S, Ctkong N, et al. Serum IL-6 and IL-1-ra with sequential organ failure assessment scores in septic patients receiving high-volume hemofiltration and continous venovenous hemofiltration. Nephrology (Carlton). 2006;11(5):386–393
- Yu C, Liu ZH, Chen ZH, Ji DX, Li LS. Improvement of monocyte fenction and immune homeostasis by high volume continuous venovenous hemofiltration in patients with severe acute pancreatitis. Int J Artif Organs. 2008;31(10):882–890
- Tao J, Gong D, Ji D, et al. Improvement of monocyte secretion function in a porcine pancreatitis model by continuous dose dependent veno-venous hemofiltration. Int J Artif Organs. 2008;31(8):716–721
- Leentjens J, Kox M, Koch RM, et al. Reversal of immunoparalysis in humans in vivo: a double-blind placebo-controlled randomized pilot-study. Am J Respri Crit Care Med. 2012;186(9):838–845
- Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int. 2006;70:1038–1045
- Garin EH, West L, Zheng W. Effect of interleukin-8 on glomerular sulfated compounds and albuminuria. Pediatr Nephrol. 1997;11(3):274–279
- Kiliś-Pstrusińska K, Zwolińska D, Medyńska A, Wawro A, Kordecki H. Interleukin-17 concentration in serum and urine of children with idiopathic nephrotic syndrome. Przegl Lek. 2006; 63(Suppl 3):198–200
- RC Wiggins. The spectrum of podocytopathies: A unifying view of glomerular disease. Kidney Int. 2007;71:1205–1214
- Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007;2:529–542

