Abstract
Acute kidney injury (AKI) is common in hematopoietic stem cell transplantation (HSCT) patients with an incidence of 21–73%. Prevention and early diagnosis reduces the frequency and severity of this complication. Predictive biomarkers are of major importance to timely diagnosis. Neutrophil gelatinase associated lipocalin (NGAL) is a widely investigated novel biomarker for early diagnosis of AKI. However, no study assessed NGAL for AKI diagnosis in HSCT patients. We performed further analyses on gathered data from our recent trial to evaluate the performance of urine NGAL (uNGAL) as an indicator of AKI in 72 allogeneic HSCT patients. AKI diagnosis and severity were assessed using Risk–Injury–Failure–Loss–End-stage renal disease and AKI Network criteria. We assessed uNGAL on days −6, −3, +3, +9 and +15. Time-dependant Cox regression analysis revealed a statistically significant relationship between uNGAL and AKI occurrence. (HR = 1.04 (1.008–1.07), p = 0.01). There was a relation between uNGAL day + 9 to baseline ratio and incidence of AKI (unadjusted HR = 1.047 (1.012–1.083), p < 0.01). The area under the receiver-operating characteristic curve for day + 9 to baseline ratio was 0.86 (0.74–0.99, p < 0.01) and a cut-off value of 2.62 was 85% sensitive and 83% specific in predicting AKI. Our results indicated that increase in uNGAL augmented the risk of AKI and the changes of day +9 uNGAL concentrations from baseline could be of value for predicting AKI in HSCT patients. Additionally uNGAL changes preceded serum Cr raises by nearly 2 days.
Introduction
Acute kidney injury (AKI) is a prevalent complication in different clinical settings particularly hematopoietic stem cell transplantation (HSCT).Citation1,Citation2 The incidence of AKI following HSCT is reported to be 21–73% and the etiology is multifactorial.Citation3 A number of studies have showed higher mortality rate in HSCT patients who had AKI.Citation4,Citation5 Prevention of AKI might reduce the frequency and severity of morbidity and mortality associated with the complication.Citation3 Besides, early recognition of AKI is essential as it allows for early interventions.Citation6 Therefore, predictive assessment tools or biomarkers are of major importance to AKI prevention or timely diagnosis.
Serum creatinine (Cr), as a conventional indicator of AKI, can be influenced by several extra-renal factors.Citation7–9 Moreover, serum Cr concentration responds slowly to kidney damage and usually increases 24–48 hours after the injurious event. Therefore, it has limited utility in the early diagnosis of AKI.Citation7,Citation8,Citation10 Neutrophil gelatinase associated lipocalin (NGAL) is among novel biomarkers which has been widely investigated for early diagnosis of AKI.Citation11–13 NGAL can be detected in the urine or plasma. In addition to early diagnosis of kidney injury, NGAL might be useful for differential diagnosis or the AKI prognosis, when compared with creatinine.Citation9,Citation14–16
Novel AKI biomarkers could be utilized in clinical settings whenever the sensitivity and specificity of appropriate cut-off thresholds have been determined. Moreover, their association with the currently recommended diagnostic criteria including of “AKI, Risk, Injury, Failure, Loss, End-stage kidney disease (RIFLE)” and “Acute Kidney Injury Network (AKIN)” should be elaborated.Citation17 Regarding NGAL, the sensitivity and specificity of this AKI biomarker have been investigated in different clinical settings. A recent systematic review and meta-analysis regarding accuracy of NGAL in diagnosis and prognosis of AKI revealed the value of this biomarker for AKI diagnosis and prognosis assessment.Citation18 The optimal thresholds of urinary or plasma NGAL for diagnosis of AKI has been suggested in critically ill and cardiac surgery patients.Citation17 However, the predictive performance of NGAL in early detection of AKI has not been examined in HSCT patients.
Hence, we analyzed our data in a recent trial on the efficacy of N-acetylcysteine for prevention of AKI in HSCT patients to evaluate the performance of urine NGAL (uNGAL) as an indicator of AKI in these patients.Citation19
Materials and methods
In our double blind, placebo controlled, randomized clinical trial, we evaluated the efficacy of N-acetylcysteine (NAC) for the prevention of acute kidney injury in patients undergoing allogeneic HSCT.Citation19 The trial was conducted at the Hematology–Oncology and Stem Cell Transplant Research Center, Tehran University of Medical Sciences (Iran), between October 2012 and September 2013. The protocol was reviewed and approved by the IRB/Ethics Committee of the institution (number: 92-02-36-22907).
AKI frequency was not different between placebo and NAC recipients and the uNGAL concentrations were similar between study groups. Therefore, data from both groups were merged for analysis (72 allogeneic HSCT recipients).
We recruited AML, ALL and MDS patients aged 15–60 years old who were admitted to receive allogeneic HSCT. Exclusion criteria were patients with hypertension, chronic kidney disease, and diabetes mellitus.
The myeloablative conditioning regimen started six days before transplantation (day −6) and included busulfan 4 mg/kg orally in divided daily doses for four days (total dose 16 mg/kg) followed by cyclophosphamide 60 mg/kg intravenously, once daily for two days (total dose 120 mg/kg). Patients received peripheral blood hematopoietic stem cells one day after completion of chemotherapy.
Prophylaxis regimens against GvHD consisted of cyclosporine (1.5 mg/kg intravenously daily from day −3 then 3 mg/kg from day +7) with a short course of methotrexate (10 mg/m2 on day +1 and 6 mg/m2 on days +3, +6, and +11). Cyclosporine trough levels were monitored weekly or if the dosing was modified. Infection prophylaxis and treatment as well as the supportive care were administered according to the institutional protocols.
Patients’ data, including age, gender, weight, height, daily serum Cr (Jaffe method) and other baseline and clinical characteristics were recorded. Diagnosis and severity of AKI were assessed based on the RIFLE and AKIN criteria during the study period (admission to day 15 after transplantation) considering daily serum Cr concentrations.Citation20,Citation21 With respect to variations of the patients’ fluid intake and output during pre- and early post-HSCT procedure according to infusion therapy, we did not use urine output in defining AKI. Baseline glomerular filtration rate was estimated using the Cockcroft–Gault equation.Citation22
In order to measure uNGAL, urine samples were collected at baseline (6 days before transplantation (−6), when the conditioning chemotherapy began), day three before transplant (day −3 when cyclosporine started as GVHD prophylaxis), day 3 after transplant (+3), day 9 after transplant (+9) and day 15 after transplant (+15). Urine samples were stored at −70°C and assayed for NGAL using the human NGAL Enzyme-linked immunosorbent assay (ELISA) kit (Biovendor-Laboratorní medicína a.s., Brno, Czech Republic) at the end of the study.
Median (range) and frequency (percentage) were reported for continuous and categorical characteristics, respectively. Kappa statistics was calculated to assess the agreement of AKIN and RIFLE definition for detecting AKI. We investigated the association of uNGAL concentrations with AKI occurrence using Cox regression model. As the increase of uNGAL concentration is suggested to be of clinical importance, we calculated the ratio of each uNGAL concentration to baseline value and possible associations with the AKI occurrence were evaluated using Cox and Logistic regression models. Receiver-operating characteristic (ROC) curves with a significant area under the curve more than 0.5 were used for definition of uNGAL cut-off points to predict AKI development. A p value < 0.05 was considered statistically significant.
Results
Summary of patients’ demographic and clinical characteristics is provided in . Twelve patients developed AKI according to the AKIN criteria while the RIFLE criteria revealed 11 AKI cases. The frequency and severity of AKI in the study patients are summarized in . There was an excellent agreement between AKIN and RIFEL criteria (Kappa values = 0.948, p < 0.001). Thus, the following analyses were performed using AKIN criteria findings. AKI occurred within 11 (−3 to 15) days after transplantation (median (minimum–maximum)). Serum Cr and uNGAL changes in patients who developed AKI are presented in .
Figure 1. Serum creatinine (Cr) and urine neutrophil gelatinase-associated lipocalin (uNGAL) concentrations changes during study period in patients who developed acute kidney injury (AKI).
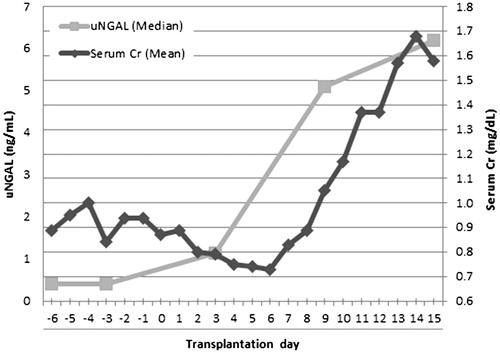
Table1. Patients’ demographic and clinical characteristics.
Table 2. Frequency and severity of acute kidney injury in study patients (n = 72).
A summary of uNGAL concentrations and ratios to baseline is described in . The distribution of uNGAL concentrations based on AKI occurrence is also illustrated (). The time-dependant Cox regression analysis revealed a statistically significant relationship between uNGAL concentrations and AKI occurrence. (HR = 1.04 (1.008–1.07), p = 0.01).
Figure 2. Natural Logarithm of urine neutrophil gelatinase-associated lipocalin (uNGAL) concentrations based on acute kidney injury (AKI) occurrence.
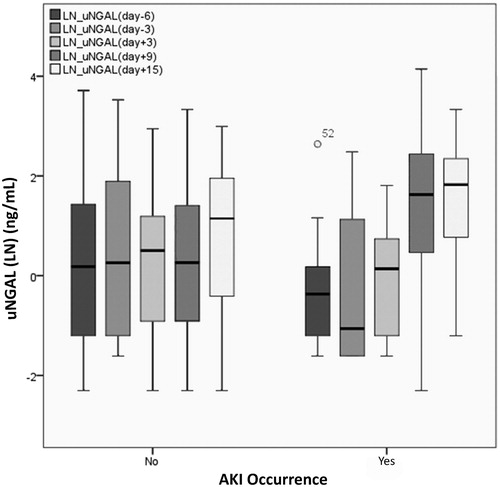
Table 3. Median (Range) of uNGAL concentrations and ratios to baseline in study patients.
Among all ratios, the day +9/baseline ratio was associated with AKI occurrence significantly (OR = 1.081 (1.010–1.157); p = 0.02). From the proportional Cox regression, there was a relation between NGAL day +9/baseline ratio and hazard of AKI (unadjusted HR = 1.047 (1.012–1.083), p < 0.01).
There were no statistically significant association between day +9/baseline ratio and each of the following variables including age (p = 0.77), disease (p = 0.92), amphotericin B deoxycholate use (p = 0.26), vancomycin use (p = 0.88), N-acetylcysteine (p = 0.65), fever occurrence (p = 0.42) and. However, we observed a significant association between gender and day + 9/baseline ratio (p = 0.04). No statistically significant relationship was found between AKI occurrence and gender (p = 0.31), age (p = 0.20), disease (p = 0.26) and complete remission status (p = 0.93).
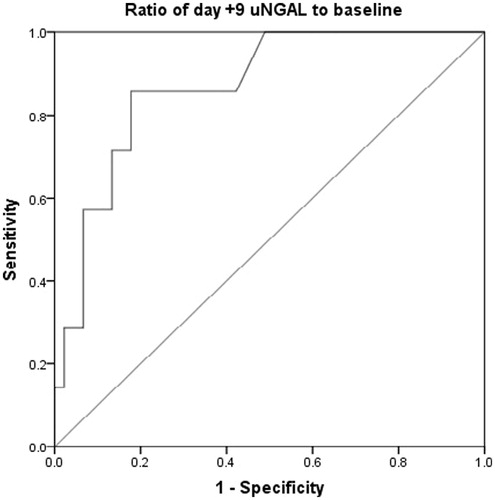
The area under the ROC curve was 0.86 (0.74–0.99, p < 0.01). A cut-off value of 2.62 for day +9/baseline ratio was 85% sensitive and 83% specific in predicting AKI ().
Discussion
Among most studied novel biomarkers of kidney injury, we chose NGAL in order to detect AKI in our study patients. In spite of limited specificity, several studies showed promising results regarding NGAL performance in early detection of AKI in comparison to other biomarkers.Citation23 Moreover, NGAL was studied in different population of patients with different etiologies of AKI including ICU patients, cancer patients specially those who received nephrotoxic medications like cisplatin and cardiac surgery.Citation23 Although using a panel of biomarkers was recommended as a better approach to diagnosis AKI, with respect to cost and other limitations we did not consider such plan in the study.Citation17,Citation23
Our results showed that one unit increase in uNGAL concentration would augment the risk of AKI by 4%. Furthermore, each one unit increase in day +9/baseline ratio would result in 8% increase in odds of AKI occurrence.
Some studies on critically ill patients, a population with indeterminate timing of AKI, showed that the admission day uNGAL could be an AKI predictive factor.Citation24 However, baseline uNGAL could not predict AKI in our study patients. Previous studies revealed conflicting results regarding baseline renal function as a risk factor of AKI in HSCT patients.Citation2 Nevertheless, none of them evaluated renal function using a novel biomarker such as NGAL.
Day +9/baseline uNGAL ratio and AKI occurrence revealed significant association. Considering the time to onset of AKI based on AKIN criteria (day +11), a temporal relationship might exist between rise of uNGAL and AKI occurrence. It is in line with recent studies that showed the rise of NGAL could be detected 24-48 hours before AKI diagnosis based on serum Cr changes.Citation25,Citation26
Considering the area under the ROC curve for day +9/baseline ratio, a cut point of 2.63 provided maximum sensitivity with an acceptable specificity. Determination of cut points for measured uNGAL in definite times with respect to AKI could be more useful in routine clinical practice. Although we could not suggest cut points for measured uNGAL on defined days, the cut point for day +9/baseline ratio has its own value. Cut off values have been suggested for uNGAL concentrations in kidney transplantation, cardiopulmonary bypass surgery, contrast-induced nephropathy, emergency and intensive care settings.Citation12 However, none of them reported a cut point for the changes of uNGAL concentrations from baseline. Moreover, an appropriate sampling time could be difficult to determine in myeloablative HSCT patients because they usually develop AKI during the first month, i.e., 7–40 days after transplant, and the exact onset of AKI is relatively unknown.Citation2,Citation25 Therefore, NGAL measurement at baseline and frequent post-transplant assessments during first month of HSCT might be essential for AKI prediction.
Urinary NGAL concentrations in our patients were relatively lower in comparison to studies in other settings.Citation18 Etiology and pathogenesis of AKI in HSCT is different from cardiopulmonary bypass surgery and contrast-induced nephropathy settings. This could be an underlying factor of diversity in uNGAL concentrations. On the other hand, some studies reported normalized uNGAL concentrations (to urine Cr). However, we used absolute uNGAL concentrations and this could be the reason for the observed difference. There is no consensus about the standard reporting practice of AKI urinary biomarkers and the normalization approach has also been criticized in the literature.Citation27–29 Besides, absolute concentration of urinary biomarkers has also shown acceptable performance for AKI diagnosis.Citation28 It should be noted that the uNGAL concentrations might have been influenced by the variations of urine volume in our study, although none of the study patients developed significant urine volume decrease and oliguria. Further studies are required to confirm range of uNGAL in HSCT patients.
In the present study, we did not use plasma NGAL because uNGAL could be more reflective of kidney injury and the sampling procedure was feasible.Citation14 A recent meta-analysis concluded that plasma/serum NGAL and uNGAL have comparable performance in the diagnosis and prognosis of AKI.Citation18 However, none of the included studies were conducted on HSCT patients; hence, the confounding effect of neutropenic fever, engraftment syndrome, graft-versus-host disease (GvHD), and sinusoidal obstruction syndrome on NGAL concentrations is unclear. Future studies should address these knowledge gaps.
We assessed uNGAL on baseline and 4 follow up time points until day +15. This pattern of uNGAL measurement might not be adequate to show a precise trend of changes. In addition, 15% of uNGAL concentrations data was missed, due to inadequate or inappropriate urine samples, which could have lowered the study power. Another caveat was the relatively short duration of study. This might have resulted in underestimation of AKI frequency. Considering our sample size and low frequency of AKI, we could not conduct subgroup analysis to evaluate the association of uNGAL with severity of AKI.
Our results indicated that increase in uNGAL concentrations augmented the risk of AKI and the changes of day +9 uNGAL from baseline could be of value for predicting AKI in HSCT patients. Additionally uNGAL changes preceded serum Cr raises by nearly 2 days. Future studies should try to confirm the predictive performance of NGAL for AKI diagnosis and prognosis in HSCT patients and to determine practical cut off values using standardized assays. Furthermore, assessment of other novel biomarkers in parallel with NGAL would add valuable information regarding early AKI diagnosis in HSCT patients.
Acknowledgments
We would like to thank the staff of BMT wards, particularly Ms Mousavi, Ms Shahriari and Ms Khalilvand, for their contribution in conducting the study.
Declaration of interest
We have nothing to declare.
References
- Parikh CR, Coca SG. Acute renal failure in hematopoietic cell transplantation. Kidney Int. 2006;69(3):430–435
- Lopes JA, Jorge S. Acute kidney injury following HCT: Incidence, risk factors and outcome. Bone Marrow Transplant. 2011;46(11):1399–1408
- Kogon A, Hingorani S. Acute kidney injury in hematopoietic cell transplantation. Semin Nephrol. 2010;30(6):615–626
- Kersting S, Koomans HA, Hene RJ, Verdonck LF. Acute renal failure after allogeneic myeloablative stem cell transplantation: Retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant. 2007;39(6):359–365
- Lopes JA, Jorge S, Goncalves S, et al. Contemporary analysis of the influence of acute kidney injury (AKI) after myeloablative hematopoietic cell transplantation on long-term patient's survival. Bone Marrow Transplant. 2008;42(2):139–141
- Hjortrup PB, Haase N, Wetterslev M, Perner A. Clinical review: Predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients. Crit Care. 2013;17(2):211
- Negi S, Shigematsu T. Current therapeutic strategies for acute kidney injury. Clin Exp Nephrol. 2012;16(5):672–678
- Bagshaw SM, Gibney RT. Conventional markers of kidney function. Crit Care Med. 2008;36(4 Suppl):S152–S158
- Carrillo-Esper R, Castillo-Albarran FM, Perez-Jauregui J. Neutrophil gelatinase-associated lipocalin: A novel biomarker in acute kidney injury. Cir Cir. 2011;79(6):577–581
- Uchino S. Creatinine. Curr Opin Crit Care. 2010;16(6):562–567
- Mori K, Mukoyama M, Nakao K. Searching for novel intercellular signal-transducing molecules in the kidney and their clinical application. Clin Exp Nephrol. 2010;14(6):523–527
- Haase-Fielitz A, Bellomo R, Devarajan P, et al. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009;24(11):3349–3354
- Moore E, Bellomo R. Novel biomarkers of acute kidney injury: Ready for clinical application? Curr Opin Crit Care. 2010;16(6):523–525
- Soni SS, Cruz D, Bobek I, et al. NGAL: A biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42(1):141–150
- Naud JF, Leblanc M. Biomarkers in acute kidney injury. Biomark Insights. 2008;3:115–125
- Haase M, Bellomo R, Haase-Fielitz A. Neutrophil gelatinase-associated lipocalin. Curr Opin Crit Care. 2010;16(6):526–532
- McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: Workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024
- Ataei S, Hadjibabaie M, Moslehi A, et al. A double-blind, randomized, controlled trial on N-acetylcysteine for the prevention of acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Hematol Oncol. 2014; [Epub ahead of print]. doi: 10.1002/hon.2141
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41
- Barrera-Chimal J, Bobadilla NA. Are recently reported biomarkers helpful for early and accurate diagnosis of acute kidney injury? Biomarkers. 2012;17(5):385–393
- Devarajan P. Review: Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology. 2010;15(4):419–428
- Zappitelli M, Washburn KK, Arikan AA, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Crit Care. 2007;11(4):R84
- McCullough PA, Bouchard J, Waikar SS, et al. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury: Executive summary from the tenth consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:5–12
- Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78(5):486–494
- Ralib AM, Pickering JW, Shaw GM, et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol. 2012;23(2):322–333
- Goldstein SL. Urinary kidney injury biomarkers and urine creatinine normalization: A false premise or not? Kidney Int. 2010;78(5):433–435