Abstract
Chronic kidney disease (CKD) is a significant public health problem and Vitamin D deficiency is prevalent in CKD and might be associated with calcium and phosphate metabolism, cardiovascular disease, infections as well as the progress of kidney dysfunction. Emerging evidence implies that Vitamin D supplements may be of benefit to CKD. Based on existing laboratory and clinical evidence, this review intends to discuss the effectiveness of Vitamin D supplements and controversy in clinical practice. The effect of Vitamin D in CKD patients is summarized in detail from CKD–mineral bone disease, the progression of renal function, cardiovascular events and immune system. Considerable disputes exist for the Vitamin D supplements in CKD, and a growing amount of experimental evidence and some clinical evidence are now gathering from in vitro, animal and epidemiological studies.
Introduction
Recent studies have focused on the role of Vitamin D supplements in the general population and special populations such as chronic kidney disease (CKD) patients,Citation1 not only because of the effects of Vitamin D on bone and mineral metabolism,Citation2 but also the significant influence on the other physiological functions: regulation of blood pressure via inhibition of the renin–angiotensin–aldosterone system (RAAS) directly;Citation3 effects on modulation of the immune system;Citation4 anti-inflammatory actionsCitation5 and regulation of insulin secretionCitation6 and lipid metabolismCitation7 due to the widespread expression of Vitamin D receptors (VDR) in many organ systems; and the potential to alter the progression of kidney disease, diminish cardiovascular disease (CVD) and even improve the prognosis of patients.
Synthesis and metabolism of Vitamin D
Vitamin D, a fat-soluble vitamin, has many forms, of which Vitamin D2 (ergocalciferol) and Vitamin D3 (cholecalciferol) are two biologically inert precursors. Vitamin D2 is mainly generated by plants via ultraviolet light irradiation from the sunlight and Vitamin D3 converts from the pre-Vitamin D3 which is synthesized with 7-dehydrocholesterol in the epidermis of animals by solar ultraviolet B radiation. Few foods are naturally rich in Vitamin D, except cod-liver oil, and only 10–20% of Vitamin D in blood circulation comes from diet and dietary supplements.Citation8 Therefore, humans derive Vitamin D mostly from skin-exposure to sunlight and may have Vitamin D deficiency without regular Vitamin D3 supplementation and daily exposure to sunlight.
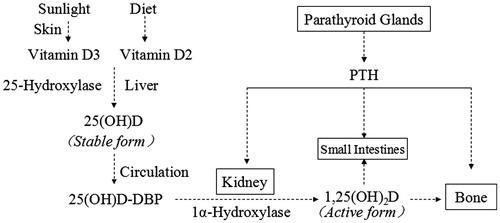
In human body, the characterization of Vitamin D is a kind of endocrine hormone.Citation9 The Vitamin D from diet could not meet the needs of the body and could be self-synthesized. In these cases, Vitamin D would not be a kind of vitamin, but a pro-hormone.Citation10 Once Vitamin D3 synthesized, it is transported with the Vitamin D-binding protein (diastolic blood pressure, DBP) to the liver where it is quickly converted to 25-hydroxyvitamin D (25(OH)D) by cytochrome P450 Vitamin D 25-hydroxylases, CYP2R1 and CYP27A1. In the circulation 25(OH)D has a strong affinity with DBP, and once binding, the complex can keep a stable state and has a longer half-life, nearly 2–3 weeks. Therefore, 25(OH)D-DBP is the main storage form of Vitamin D and commonly used for monitoring the body’s Vitamin D status.Citation11 After glomerular filtration, the 25(OH)D is converted to 1.25-dihydroxyvitamin D (1,25(OH)2D) by renal 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) in the proximal tubular cells. This is the hormonally active form of Vitamin D that acts on VDR in different target organs.Citation12 As a biologically active form, its half-life is short, only 8–12 h, which provides essentially no information about the body’s nutritional Vitamin D status.Citation13 The 1,25(OH)2D synthesis in the kidney is affected by diverse regulatory hormones and genetic factors,Citation14 such as parathyroid hormone (PTH), fibroblast growth factor 23 (FGF-23), calcium phosphorus metabolism. The active Vitamin D can further be degraded to an inactive state by the renal enzyme 24-hydroxylase (CYP24A1) ().
Vitamin D deficiency/insufficiency in CKD
Metabolism of Vitamin D in CKD
Kidneys as the mainly organs convert the 25(OH)D to the active forms of Vitamin D, 1,25(OH)2D. The Vitamin D deficiency or insufficiency is prevalent in patients with CKD which can decline renal production of 1,25(OH)2D.Citation15 There are several mechanisms for the dysregulation of Vitamin D metabolism during the progress of CKD.Citation16 The limitation of 25(OH)D-DBP in the circulation is not only because of the reduced glomerular filtration rates (GFR),Citation17 but also due to the loss of protein into the urine,Citation18 that restrict the production of 1,25(OH)2D which is one of the most active hormonal and a pleiotropic metabolite in human. FGF-23 as a counter-regulatory phosphaturic hormone for Vitamin D is another key factor which increase early in CKD, contributing by directly suppressing the activity of 1,25(OH)2D via inhibition of 1-α-hydroxylase and degradation of 1,25(OH)2D via the stimulation of 24-hydroxylase.Citation19 Carboxyl (C)-terminal fragments of PTH may have potential suppressive effects on 1,25(OH)2D synthesis.Citation20 Since the altering of FGF-23 and the PTH levels involves in the progression of CKD, Vitamin D insufficiency or deficiency could be the consequence of CKD as well the cause of CKD.Citation21
The diagnosis of Vitamin D deficiency
Serum 25(OH)D is commonly recognized as a valuable biomarker to roughly reflect the patient’s nutritional Vitamin D status.Citation22 At present there is no unified criterion to define Vitamin D deficiency because most of the evidence for Vitamin D comes from population studies. These suggest a positive relation between high blood levels of the vitamin and a reduced risk of disease without providing explanations. The International Osteoporosis Foundation suggests that serum 25(OH)D levels should reach up to 75 nmol/L (30 ng/mL) to suppress the maximum of PTH.Citation23 The American Geriatrics Society published a consensus that serum 25(OH)D levels about <75 nmol/L (30 ng/mL) could increase the risk of falls especially among older populations.Citation24 However, United States Institute of Medicine reported that Vitamin D deficiency at serum 25(OH)D levels between <50 nmol/L (20 ng/mL) and under 75 nmol/L (30 ng/mL) is considered an insufficient amount.Citation25 Some experts suggested that serum 25(OH)D levels above 30 ng/mL could be desirable for optimal health and above 50 ng/mL should raise potential adverse effects, such as increased the risk for pancreatic cancer. This is proved by some studies, that revealed that people with the highest Vitamin D levels seemed to have a higher, rather than lower,Citation26 chance of developing breast cancer.Citation27 Otherwise one study concluded that higher 25(OH)D levels were associated with significantly reduced mortality in patients with colorectal and breast cancer.Citation28 Therefore, there is no consensus on a cut-off point to define Vitamin D deficiency and the serum levels of 25(OH)D signifying deficiency or sufficiency are disease dependent, moreover the specific therapeutic levels for Vitamin D sufficiency in different diseases and the dose–response characteristics of Vitamin D will ensure progress in research, clinical medicine and public health.Citation29
There are increasing epidemiological data published in recent years suggesting that Vitamin D deficiency is commonly found in the general population, especially prevalent in CKD patients. Based on the key statistics from the National Health and Nutrition Examination Survey (NHANESIII), the prevalence of 25(OH)D levels <20 ng/mL among adults with stages 1–3 CKD is 9–14%. Among adults with estimated GFR (eGFR) <30 mL/min/1.73 m2, more than 27% had 25(OH)D deficiency.Citation30 Furthermore, nearly 80% of dialysis patients had Vitamin D deficiency or insufficiency (<30 ng/mL).Citation31–33 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines for Bone Metabolism and Disease in CKD recommends that serum level of 25(OH)D <75 nmol/L (30 ng/mL) should be considered to provide prevention and treatment in patients with stages 3 and 4 CKD.Citation34 The treatment of Vitamin D insufficiency or deficiency when present in non-CKD patients according to corresponding meta-analysis is warranted since such therapy may reduce or prevent secondary hyperparathyroidism (SHPT) and decrease the incidence of hip fractures.Citation29
Vitamin D supplements and CKD
Vitamin D and derivatives
Vitamin D and derivatives have been widely used in a variety of medical areas, especially in the management of CKD, for example, the control of hyperparathyroidism.Citation3 The different Vitamin D analogs have differential effects on physiological function, which could be classified by the activity.Citation35 Nutritional Vitamin DCitation25 since they can be supplemented by oral intake of vitamin D rich or fortified diets is non-biological active form such as ergocalciferol, cholecalciferol and traditionally the active forms, 1,25(OH)2D, known as hormone are calcitriol and alfacalcidol. More recently, four Vitamin D analogs have been introduced in the nephrology area and play an increasingly important role in CKD treatment which is doxercalciferol, paricalcitol, oxacalcitriol and falecalcitriol.Citation36 Vitamin D receptor activator (VDRA) for any compound can activate VDR.Citation37 The different forms of Vitamin D compounds are listed in .
Table 1. Vitamin D and derivatives.
Vitamin D supplement in chronic kidney disease–mineral bone disease
Mineral bone disease as a systemic syndrome, contributing to the pathogenesis of renal osteodystrophy, is early and common complications in early stages of CKD associated with an increased risk of cardiovascular calcification and mortality although the rate of change and severity of abnormalities is highly variable among patients.Citation38 CKD patients with alteration in Vitamin D metabolism cannot make enough l,25(OH)2D to keep their bones mineralized normality, have abnormal calcium–phosphorus metabolism leading to SHPT which associated with an increased risk of cardiovascular calcification and mortality.Citation2 All these causes have significant correlation with prognosis.Citation39 The KDOQI guidelines have suggested that the treatment with nutritional or active Vitamin D is according to the target range of intact plasma PTH for the stage of CKD. In the dialysis population, the PTH target focuses on avoidance of risk at extremes of PTH, which is 2–9 times of the upper limit of the normal reference range.Citation40 It suggests that serum levels of calcium, phosphate, alkaline phosphatase, PTH and 25(OH)D in patients with CKD stages 4 ando 5 should be monitored with a frequency based on stage, rate of progression and whether specific therapies have been initiated to avoid adverse reactions.Citation41
Vitamin D supplement and the progression of renal function in CKD
Emerging epidemiological studies have shown that Vitamin D deficiency may be associated with the progression of renal function in CKD.Citation42 Previously, Melamed et al. suggested that after multivariable adjustment, participants with 25(OH)D levels <15 ng/mL were 2.6 times more likely to progress to end stage renal disease (ESRD) compared with participants with higher 25(OH)D levels.Citation43 A research from USA enrolling 5888 participants showed that each 10 ng/mL lower 25(OH)D was associated with a 25% greater risk of rapid GFR loss [95% confidence interval (CI) 5%, 49%, p = 0.0]), adjusting for potential confounding characteristics and low 25(OH)D concentration was associated with eGFR loss with a magnitude similar to or stronger than that for diabetes or hypertension, the most important traditional risk factors for CKD.Citation44 Albuminuria as an important marker and major risk factor for progressive decline in renal function is considered by many to be the first step in an inevitable progression to nephropathy and renal failure. Multiple studies have shown an inverse relationship between the level of Vitamin D and degree of albuminuria.Citation45 NHANES III, in which 15,068 adults participating, showed that the patients with higher albuminuria were observed with lower Vitamin D.Citation46 These findings suggest that low 25(OH)D concentration may be a modifiable risk factor for the progression of CKD.
Vitamin D supplement and proteinuria
Treatment with Vitamin D analogs for patients with proteinuric renal diseases could be helpful because Vitamin D has a very important role in regulating systems that could be important in the pathobiological state of proteinuria.Citation47 Fishbane et al. carried out a double-blind randomized study recruited 61 patients with CKD and concluded that oral paricalcitol can decrease urinary protein–creatinine ratios (UACR),Citation48 and plasma concentration of PTH level, C-reactive protein and TGF-β were reduced significantly, additional.Citation49 The Vitamins and Lifestyle (VITAL) study (NCT00421733), the multinational, placebo-controlled, double-blind trial, evaluated the anti-albuminuric effect of paricalcitol 1 or 2 µg/day versus placebo in 281 patients with type 2 diabetes and albuminuria who were receiving angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers to control blood pressure and proteinuria and found that patients on 2 µg/day paricalcitol showed an early, sustained reduction in UACR, ranging from −18% to −28% (p = 0.014 vs. placebo).Citation50 Furthermore, in a small group analysis, the effect of calcitriol on urinary protein excretion in patients with immunoglobulin A nephropathy was determined;Citation51 Agarwal et al. reported that paricalcitol also has an anti-proteinuric effect on patients with CKD;Citation52 recently a systematic review addressed that active Vitamin D analogs such as paricalcitol and calcitriol reduce residual proteinuria.Citation53 These researches indicated that Vitamin D supplement can reduce urinary protein in patients with CKD, and therefore it may exert protective effects on progression of renal function. But so far, there is no exact clinical evidence showing active Vitamin D has the effect in retarding the progression of renal function.
Vitamin D supplement and blood pressure
Mounting evidence from clinical studies has demonstrated an inverse relationship between serum Vitamin D levels and the blood pressure. Prospective studies with cohorts from the Health Professionals’ Follow-Up Study and the Nurses’ Health Study during 4 years of follow-up also showed that the multivariable relative risk of incident hypertension among patients whose measured plasma 25(OH)D levels were <15 ng/mL (i.e., Vitamin D deficiency) was three times compared with those whose levels were ≥30 ng/mL;Citation54 women with low Vitamin D levels (17 ng/mL or 42 nmol/L) had a 67% increased risk of developing hypertension.Citation55 These studies implied that high blood pressure might benefit from Vitamin D supplement. A study was consisted of 148 women with 8 weeks of supplementation with Vitamin D3 (cholecalciferol) and calcium, and the supplementation resulted in a decrease in systolic blood pressure (SBP) of 9.3% (p = 0.02) and a decrease in serum PTH of 17% (p = 0.04).Citation56,Citation57 In the VITAL study, patients with high-dose 2 µg/day paricalcitol showed SBP with 3–9 mmHg reduction compared with placebo.Citation50 Therefore, Vitamin D may reduce the risk of hypertension and lower blood pressure. However, in meta-analyses of 10 trials, supplementation no significantly reduced SBP and did not affect DBP.Citation58 Thus, here is no consistent result in existing clinical trials giving confirmed evidence that Vitamin D supplementation may not have blood pressure benefits. Further research is needed to confirm these results.
Clinical and epidemiological studies have suggested that Vitamin D analog may suppress proteinuria and hypertension; however, there is no evidence from controlled clinical trial data. In a meta-analysis of subgroup analysis including four small clinical studies, data indicated that Vitamin D (paricalcitol, maxacalcitol, doxercalciferol) cannot reduce the risk of ESRD and Vitamin D therapy may be independently associated with reduced mortality in CKD.Citation59 Therefore, more high quality controlled clinical trials with clinical endpoints as ESRD are needed to prove that Vitamin D supplement could attenuate the progression of renal function directly.
Laboratory studies indicate that Vitamin D analogs suppress the renin–angiotensin system (RAS) and inhibit production of inflammatory cytokines for improving the renal function. In animal models studies, Li et al. found that mice with Vitamin D deficiency elevated production of renin as well as in cell culture, whereas injection of 1,25(OH)2D reduces renin synthesis which is independent of calcium metabolism.Citation60 Furthermore, they have found that mice lacking the VDR increased renin synthesis and angiotensin (Ang) II, leading to hypertension, cardiac hypertrophy.Citation61 In addition, the 5/6 nephrectomy uremic rat model treated with an ACEI and a paricalcitol, suppressed urinary protein excretion, reduced both mesangial cell proliferation and the degree of glomerulosclerosis via mediation of the TGF-β signaling pathway and matrix-regarding molecules and this effect was amplified when blood pressure was controlled via RAAS blockade.Citation62 Tan et al. demonstrated that the Vitamin D analog paricalcitol ameliorates renal interstitial fibrosis in a mouse model of obstructive nephropathy.Citation63 Thus, these evidence supported that the potential renoprotective effects of Vitamin D supplement include suppression of the RAAS and a reduction in proteinuria through negative regulation of the RAAS and directly the protection of glomerulus via the activation of VDR and anti-proliferation, pro-differentiation and anti-inflammation which may be attributed to a suppression of the NF-κB pathway promoting both inflammation and fibrogenesis by regulating gene expression of cytokines, chemokines and adhesion molecules including interleukin-6 (IL-6), MCP-1 and tumor necrosis factor-α (TNF-α).Citation64
Vitamin D supplement and cardiovascular events in CKD
Animal experiments demonstrated that VDR knockout mice can develop cardiac hypertrophy and high blood pressure due to excessive stimulation of the RAS and spontaneously hypertensive rats,Citation61 treated with paricalcitol or doxercalciferol, have the reduction in left ventricular hypertrophy (LVH), attenuation of cardiac and cardiomyocyte hypertrophy, improvement of left ventricular diastolic function, and suppression of episodes of heart failure.Citation65
Clinical and epidemiological studies have found that Vitamin D deficiency/insufficiency has been recently associated with important CVD risk factors such as obesity, hypertension, and diabetes mellitus in CKD and in the general populationCitation66 and inversely associated with risk for developing coronary artery calcification.Citation67 A study with 1739 participants reported that individuals with Vitamin D deficiency (25(OH)D < 15 ng/mL) had 1.62 times increased in incident cardiovascular events compared with those without Vitamin D deficiency, and the effect was evident in participants with hypertension.Citation68 But Mehrotra et al. analyzed CKD patients from NHANESIII that low serum Vitamin D predicts a higher mortality and has no significant with cardiovascular events in CKD patients undergoing dialysis.Citation69 Therefore, Vitamin D might be a determinant of cardiovascular mortality, but without cogent evidence directly related to cardiovascular endpoints.
Recently, convincing data demonstrate the effect of Vitamin D supplementation in the benefit of the cardiovascular system and reduction for any cardiovascular event. Active Vitamin D can attenuate myocardial hypertrophy in hemodialysis patients with SHPT.Citation70 A meta-analysis of prospective studies performed by Wang et al. showed that Vitamin D supplements may reduce the risk for cardiovascular events and improve cardiovascular health in adults, especially in dialysis patients.Citation71 However, a surprise finding came from the Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity (PRIMO) trial (NCT00497146): it was a double-blind, randomized placebo-controlled trial among 227 patients with pre-dialysis patients with mild to moderate left ventricular hypertrophy and preserved left ventricular ejection fraction was randomly assigned to receive 2 μg/day of oral paricalcitol (115 patients) or placebo (112 patients) for 48 weeks; left ventricular mass index, echocardiographic changes in left ventricular diastolic function, CVD events leading to hospitalization or death and change in cardiac biomarkers were observed and analyzed, and the results showed the surprise finding that 48 weeks therapy with paricalcitol did not alter left ventricular mass index or improve certain measures of diastolic dysfunction in patients but who had fewer hospitalizations for CVD events.Citation72 These results differ from reports of Vitamin D therapy in animal models and human observational data. One potential explanation for the results is that Vitamin D might be associated with a reduction in mortality among patients on dialysis, but PRIMO study did not include patients on dialysis. The other explanation is FGF23, which was mentioned in “Metabolism of Vitamin D in CKD”. FGF23 as a hormone that regulates phosphate which might be increased by paricalcitol is related with mortality and cardiovascular events from many epidemiological data in CKD patients.Citation73 Therefore, further randomized, controlled trials are needed to conclusively determine whether Vitamin D supplementation reduces CVD, CVD-related morbidity and all-cause mortality in CKD.
Vitamin D supplement and immune system in CKD
It is well known that CKD is a state of microinflammation, which might be contributed by Vitamin D deficiency besides the uremic milieu.Citation74 Vitamin D deficiency is associated with dysfunction of both innate and adaptive immunity in patients with CKD who have abnormal inflammatory response, increased susceptibility to infection and increased prevalence of malignancies.Citation75 Some studies unraveled that treatment with Vitamin D can alter immune function in CKD patients, enhance Th2 cell differentiation,Citation76 decrease IL-6, IL-8, IL-1 and TNF-α expression,Citation77 attenuate oxidative stress, even reduce platelet activating factor/thrombin activity and metabolism in hemodialysis patients.Citation78 Therefore, the immune regulatory function of Vitamin D supplement is well evaluated, associated with a decreased risk of infection, as well as reduced all-cause mortality. The study “Dialysis Infection and Vitamin D in New England” DIVINE is a on-going randomized, placebo-controlled trial (NCT00892099) could provide a well understanding of the immune regulatory function of vitamin D in hemodialysis patients via evaluating cathelicidin, cytokine levels, and the incidence of infections in future.
Controversy for vitamin D in CKD
Controversy remains regarding the causality between Vitamin D deficiency and poor prognosis for patients with CKD. Patients who have serious condition could have low Vitamin D levels due to lack of adequate daylight outdoor exposure, malnutrition and abnormal Vitamin D metabolism.Citation79 A notable factor concerned is the socioeconomic status. In developing countries, the prevalence of Vitamin D deficiency varies widely by and within regions, and the prevalence ranges between 30% and 90%.Citation80 Lack of large-scale, epidemiologic studies and heterogeneity with respect to genetic, nutritional, lifestyle and socioeconomic status accounts for this variability. Based on the evidence from the LUdwigshafen Risk and Cardiovascular Health study, some researchers presumed that many various parameters might contribute to a poor Vitamin D status, and Vitamin D deficiency might only be a sign of no health status, rather than the direct cause of poor prognosis in patients with CKD.Citation81
It remains to be established that optimal serum concentrations of Vitamin D for bone and general health in different diseases which would be variety depending on the different physiological states. Currently, the optimal Vitamin D status, >30 ng/mL (75 nmol/L) in CKD depends on the effect for preventing a rise in iPTH levels.Citation82 But a serum concentration of 100 nmol/L Vitamin D appears to reduce the risk of hip and other nonvertebral fractures.Citation83 Actually, the establishment of an optimal Vitamin D should also consider the non-calcemic effects of Vitamin D that effect on VDR, resulting in inhibition of RAS, suppression of inflammatory reaction and modulation of immune system.Citation84
Increasing confusion exists as to which Vitamin D compounds are more appropriate for persons with CKD. The controversy about nutritional Vitamin D and active Vitamin D heats up. Some opinion-based guidelines recommend administration of such nutritional Vitamin D agents as ergocalciferol or cholecalciferol as the first therapy in hyperparathyroidism associated with low circulating levels of 25(OH)D (<30 ng/mL) in non-dialysis dependent CKD patients.Citation85 The deficiency of serum 25(OH)D can increase 1-α hydroxylation secretion in many extrarenal sites as a compensation for loss. Dusso et al. reported that 1,25(OH)2D deficiency, but not hyperparathyroidism, may play a role in the stimulation of 1,25(OH)2D production by macrophages which display higher rates of 1,25(OH)2D synthesis and lower rates of catabolism in chronic renal failure.Citation86 Several studies have concluded the potential benefits of ergocalciferol in CKD, including satisfactory to inadequate lowering of PTH level to target ranges, improving response to erythropoietin stimulating agents, and salutary effects on glycemic controls, even suppress the risk of infection by increasing cathelicidin.Citation59,Citation87,Citation88 Compared with nutritional Vitamin D agents, active Vitamin D compounds appear to more effectively lower the circulating levels of alkaline phosphatase, and PTH in CKD patients.Citation89,Citation90 Epidemiologic studies have almost consistently indicated the survival benefit of active Vitamin D agents across all stages of CKD, including among dialysis patients with 25(OH)2D deficiency, for example, proteinuria decrease, improvement of the cardiovascular system and the risk of death reduction.Citation37
In addition, the controversy surrounding the potential clinical applications of new Vitamin D derivatives and classical active Vitamin D is discussed in many researches.Citation91–93 VDRA analogs have been developed with enhanced affinity for the VDR and diminishing or even abolishing the calcemic and phosphatemic effects,Citation36 while calcitriol appears to be more potent than paricalcitol in mobilizing calcium resorption from bone and was thought to be associated with increasing serum calcium level resulting in hypercalcemia.Citation91 Recently, in an investigator-initiated multicenter randomized clinical trial, researchers reported that there was no difference between alfacalcidol and paricalcitol in the treatment of SHPT in hemodialysis patients.Citation93 As a result, there has been increasing confusion as to whether Vitamin D agents are more or less appropriate for CKD patients or which type nutritional versus active Vitamin D preparations should be used.Citation37 Thus, the effects of different forms of Vitamin D may differ from metabolism and mechanisms which should cause practice attention in clinical.
Despite considerable disputes exist for the Vitamin D supplements in CKD, a growing amount of experimental evidence and some clinical evidence are now gathering from in vitro, animal, and epidemiological studies and suggesting that Vitamin D supplements may be of benefit to CKD patients. However, current guidelines only recommend Vitamin D treatment in patients with moderate CKD accompanied by SHPT and Vitamin D insufficiency. The ideal Vitamin D therapy for CKD patients should be the one that improves survival irrespective of suggested or imposed target ranges for arbitrary or opinion-based surrogate endpoints.Citation37 Consequently, the randomized clinical trials, in which are ongoing, are needed to determine whether Vitamin D supplementation could reduce future CVD events, the rate of progression of kidney disease and mortality risk in individuals with CKD as well more accurately define the precise therapeutic agent, dose, timing, monitoring parameters and indications for Vitamin D therapy.
Declaration of interest
No conflict of interest exits in the submission of this manuscript.
References
- Handelman GJ, Levin NW. Guidelines for vitamin supplements in chronic kidney disease patients: What is the evidence? J Ren Nutr. 2011;21:117–119
- Nigwekar SU, Tamez H, Thadhani RI. Vitamin D and chronic kidney disease–mineral bone disease (CKD–MBD). Bonekey Rep. 2014;3:498. doi: 10.1038/bonekey.2013.232
- Martin KJ, Gonzalez EA. Vitamin D supplementation in CKD. Clin Nephrol. 2011;75:286–293
- Sterling KA, Eftekhari P, Girndt M, Kimmel PL, Raj DS. The immunoregulatory function of vitamin D: Implications in chronic kidney disease. Nat Rev Nephrol. 2012;8:403–412
- Donate-Correa J, Dominguez-Pimentel V, Mendez-Perez ML, et al. Selective vitamin D receptor activation as anti-inflammatory target in chronic kidney disease. Mediat Inflamm. 2014;2014:670475
- Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385
- Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012;108:1915–1923
- Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820
- Bouillon R, Okamura WH, Norman AW. Structure–function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S
- Santoro D, Caccamo D, Gagliostro G, et al. Vitamin D metabolism and activity as well as genetic variants of the vitamin D receptor (VDR) in chronic kidney disease patients. J Nephrol. 2013;26:636–644
- Hollis BW. Assessment and interpretation of circulating 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in the clinical environment. Endocrinol Metab Clin North Am. 2010;39:271–286, table of contents
- Apukhovs'ka LI. physiologic functions of vitamin D3 and its metabolism in the body in normal states and in some pathologies. Ukr Biokhim Zh. 2000;72:138–146
- Hamano T. Vitamin D deficiency in CKD patients. Clin Calcium. 2007;17:718–724
- Echida Y, Mochizuki T, Uchida K, Tsuchiya K, Nitta K. Risk factors for vitamin D deficiency in patients with chronic kidney disease. Intern Med. 2012;51:845–850
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38
- Rouached M, El Kadiri Boutchich S, Al Rifai AM, Garabedian M, Fournier A. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2008;74:389–390
- Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435
- Usatii M, Rousseau L, Demers C, et al. Parathyroid hormone fragments inhibit active hormone and hypocalcemia-induced 1,25(OH)2D synthesis. Kidney Int. 2007;72:1330–1335
- de Boer IH, Thadhani R. Vitamin D deficiency: Consequence or cause of CKD? Clin J Am Soc Nephrol. 2013;8:1844–1846
- Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: Physiology and biomarkers. Am J Clin Nutr. 2008;88:500S–506S
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: Vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–1154
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society consensus statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147–152
- Bhan I, Hewison M, Thadhani R. Dietary vitamin D intake in advanced CKD/ESRD. Semin Dial. 2010;23:407–410
- Skinner HG, Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev. 2006;15:1688–1695
- Scarmo S, Afanasyeva Y, Lenner P, et al. Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: A nested case–control study. Breast Cancer Res. 2013;15:R15
- Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: A review. Cancer Epidemiol Biomarkers Prev. 2006;15:1427–1437
- Spedding S, Vanlint S, Morris H, Scragg R. Does vitamin D sufficiency equate to a single serum 25-hydroxyvitamin D level or are different levels required for non-skeletal diseases? Nutrients. 2013;5:5127–5139
- Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis d in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–1151
- Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013
- Mailliez S, Shahapuni I, Lecaque C, Massy ZA, Choukroun G, Fournier A. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2008;74:389; author reply 389
- Bhan I, Burnett-Bowie SA, Ye J, Tonelli M, Thadhani R. Clinical measures identify vitamin D deficiency in dialysis. Clin J Am Soc Nephrol. 2010;5:460–467
- Orita H, Akizawa T. Outlines of K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Clin Calcium. 2004;14:693–697
- Galassi A, Bellasi A, Auricchio S, Papagni S, Cozzolino M. Which vitamin D in CKD–MBD? The time of burning questions. Biomed Res Int. 2013;2013:864012
- Slatopolsky E, Finch J, Brown A. New vitamin D analogs. Kidney Int Suppl. 2003;63:S83–S87
- Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1529–1539
- Fujii H. CKD–MBD (chronic kidney disease–mineral and bone disorder). Effect of vitamin D on kidney and cardiovascular system. Clin Calcium. 2010;20:1045–1050
- Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin Proc. 2010;85:752–757; quiz 757–758
- Olgaard K, Lewin E, Silver J. Calcimimetics, vitamin D and advance in the management of CKD–MBD. Nephrol Dial Transplant. 2011;26:1117–1119
- Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP. Kidney bone disease and mortality in CKD: Revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int Suppl. 2010;117:S10–S21
- Williams S, Malatesta K, Norris K. Vitamin D and chronic kidney disease. Ethn Dis. 2009;19:S5–S8–S11
- Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20:2631–2639
- de Boer IH, Katz R, Chonchol M, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2011;6:2141–2149
- Inda Filho AJ, Melamed ML. Vitamin D and kidney disease: What we know and what we do not know. J Bras Nefrol. 2013;35:323–331
- de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the third national health and nutrition examination survey (NHANES III). Am J Kidney Dis. 2007;50:69–77
- Agarwal R. Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin J Am Soc Nephrol. 2009;4:1523–1528
- Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N. Oral paricalcitol in the treatment of patients with CKD and proteinuria: A randomized trial. Am J Kidney Dis. 2009;54:647–652
- Alborzi P, Patel NA, Peterson C, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension. 2008;52:249–255
- de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (vital study): A randomised controlled trial. Lancet. 2010;376:1543–1551
- Liu LJ, Lv JC, Shi SF, Chen YQ, Zhang H, Wang HY. Oral calcitriol for reduction of proteinuria in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis. 2012;59:67–74
- Agarwal R, Hynson JE, Hecht TJ, Light RP, Sinha AD. Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int. 2011;80:1073–1079
- de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ. Active vitamin D treatment for reduction of residual proteinuria: A systematic review. J Am Soc Nephrol. 2013;24:1863–1871
- Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069
- Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832
- Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118
- Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637
- Pittas AG, Chung M, Trikalinos T, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314
- Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2009;4:CD008175
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin–angiotensin system. J Clin Invest. 2002;110:229–238
- Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin–angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132
- Mizobuchi M, Morrissey J, Finch JL, et al. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol. 2007;18:1796–1806
- Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393
- Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of nf-kappab signaling. J Am Soc Nephrol. 2008;19:1741–1752
- Kong J, Kim GH, Wei M, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol. 2010;177:622–631
- Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the united states: Data from the third national health and nutrition examination survey. Arch Intern Med. 2007;167:1159–1165
- de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-Hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–1812
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511
- Mehrotra R, Kermah DA, Salusky IB, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the united states. Kidney Int. 2009;76:977–983
- Park CW, Oh YS, Shin YS, et al. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33:73–81
- Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323
- Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The primo randomized controlled trial. JAMA. 2012;307:674–684
- Canziani ME, Tomiyama C, Higa A, Draibe SA, Carvalho AB. Fibroblast growth factor 23 in chronic kidney disease: Bridging the gap between bone mineral metabolism and left ventricular hypertrophy. Blood Purif. 2011;31:26–32
- Merino A, Nogueras S, Buendia P, et al. Microinflammation and endothelial damage in hemodialysis. Contrib Nephrol. 2008;161:83–88
- Lang CL, Wang MH, Chiang CK, Lu KC. Vitamin D and the immune system from the nephrologist's viewpoint. ISRN Endocrinol. 2014;2014:105456
- Lang CL, Wang MH, Hung KY, Chiang CK, Lu KC. Altered molecular repertoire of immune system by renal dysfunction in the elderly: Is prediction and targeted prevention in the horizon? EPMA J. 2013;4(1):17. doi: 10.1186/1878-5085-4-17
- Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–361
- Bucharles S, Barberato SH, Stinghen AE, et al. Impact of cholecalciferol treatment on biomarkers of inflammation and myocardial structure in hemodialysis patients without hyperparathyroidism. J Ren Nutr. 2012;22:284–291
- Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: A meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374–382
- Arabi A, El Rassi R, El-HajjFuleihan G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat Rev Endocrinol. 2010;6:550–561
- Winkelmann BR, Marz W, Boehm BO, et al. Rationale and design of the LURIC study – a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics. 2001;2:S1–S73
- Holick MF. Optimal vitamin D status for the prevention and treatment of osteoporosis. Drugs Aging. 2007;24:1017–1029
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716
- Arenas MD, Alvarez-Ude F, Torregrosa V, et al. Consequences of the implementation of K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease in a population of patients on chronic hemodialysis. J Nephrol. 2007;20:453–461
- Dusso AS, Finch J, Brown A, et al. Extrarenal production of calcitriol in normal and uremic humans. J Clin Endocrinol Metab. 1991;72:157–164
- Alvarez J, Wasse H, Tangpricha V. Vitamin D supplementation in pre-dialysis chronic kidney disease: A systematic review. Dermatoendocrinology. 2012;4:118–127
- Kandula P, Dobre M, Schold JD, Schreiber MJ Jr, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6:50–62
- Bhan I, Camargo CA, Jr Wenger J, et al. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol. 2011;127:1302–1304, e1301
- Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2009;4:CD005633
- Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63:1483–1490
- Hansen D, Rasmussen K, Pedersen SM, Rasmussen LM, Brandi L. Changes in fibroblast growth factor 23 during treatment of secondary hyperparathyroidism with alfacalcidol or paricalcitol. Nephrol Dial Transplant. 2012;27:2263–2269
- Moe S, Wazny LD, Martin JE. Oral calcitriol versus oral alfacalcidol for the treatment of secondary hyperparathyroidism in patients receiving hemodialysis: A randomized, crossover trial. Can J Clin Pharmacol. 2008;15:e36–e43