Abstract
Aim: The clinical course and outcome of children with thrombotic thrombocytopenic purpura and posterior reversible encephalopathy has not been observed and studied till date. The aim of the present study was to know the clinical course and outcome of children with thrombotic thrombocytopenic purpura and posterior reversible encephalopathy. Results from our observation invite potential insight for further research on this subject. Methods: From January 2005 to February 2013, seven children diagnosed with thrombotic thrombocytopenic purpura and posterior reversible encephalopathy syndromes were admitted in a tertiary care hospital in Srinagar, Kashmir. The demographic parameters, clinical characteristics and laboratory data were noted. The outcome was defined in the form of complete recovery or death. Thrombotic thrombocytopenic purpura was diagnosed on clinical grounds, laboratory parameters and renal biopsy. The diagnosis was established after an expert opinion from a hematologist. Posterior reversible encephalopathy syndrome was defined on neuroimaging. Results: The common clinical parameters which were shared by all the patients were hypertension and altered sensorium. Four (57.1%) patients showed clinical deterioration and died within one week of admission even after intensive management. Three (42.8%) patients improved clinically and recovered fully and were discharged in stable clinical condition. Repeat imaging on discharge was normal. Conclusion: This series of seven pediatric patients is the first series on this subject. The presence of posterior reversible encephalopathy syndrome in pediatric patients with thrombotic thrombocytopenic purpura complicates the clinical course and worsens the prognosis.
Introduction
Thrombotic thrombocytopenic purpura (TTP) not infrequently presents with reversible neurological manifestations which include headache, confusion, ataxia, seizures, altered mental status and focal abnormalities.Citation1 Most of the neurological manifestations in TTP are reversible.Citation2 Posterior reversible encephalopathy syndrome (PRES) has been reported to be a predominant neurological abnormality in TTP in adults.Citation2 However, it is fairly uncommon in children. TTP when uncomplicated carries a good prognosis especially in adults as compared to children.Citation1 However, complications like renal failure worsen the prognosis. The pathophysiological mechanisms and neuroimaging abnormalities in PRES with TTP are different from those which occur in PRES without TTP. The transient neurological abnormalities in TTP could be explained by microthrombi causing ischemic brain damage which are largely reversible.Citation3 PRES is characterized by transient neurological symptoms, including headache, altered mental status, seizures and visual impairment occurring in some patients hospitalized for acute illness.Citation4 Neuroradiological study usually shows edema involving the cerebral posterior regions.Citation5–7 The most common causes of PRES are hypertensive encephalopathy, preeclampsia, eclampsia, and cyclosporine A (CSA) neurotoxicity.Citation8,Citation9 Other less-defined conditions in children include nephrotic syndrome, acute nephritis, hemolytic uremic syndrome and systemic lupus erythematosus. The presence of TTP as a cause of PRES has been described but not very well established.Citation3 The distinctiveness of PRES is its reversibility both in terms of the clinical and radiological abnormalities after institution of appropriate treatment and removal of the precipitating factors. Although PRES is reversible once treatment is instituted, delayed diagnosis and therapy can result in permanent brain damage and neurological sequelae, such as chronic epilepsy.Citation5,Citation6 The occurrence of PRES in TTP pediatric patients and its effect on the outcome has not been studied. We tried to evaluate the clinical course, study the laboratory parameters and observe the outcome in children admitted with TTP and PRES.
Material and methods
Between January 2005 and February 2013, seven patients in the age range 5–18 years were admitted in a tertiary care hospital at Srinagar. All the seven patients were managed in the ICU. Patient data were collected at the time of admission and during hospital stay. This study was approved by hospital ethical committee. Exclusion criteria include adult patients and those who did not have adequate workup. The data collected include patient demographics, clinical characteristics, laboratory parameters which include total leukocyte count, platelet count, peripheral blood film, coagulation profile, kidney function tests, liver function tests, urine examination and cultures. Other biochemical parameters like LDH and fibrinogen levels were also noted. We staged hypertension as per fourth task force report on high blood pressure in children and adolescents by comparing patients’ BP reading with the BP level for boys and girls by age and height percentile in BP table prepared by National Heart Lung and Blood Institute (NHLBI).Citation10 Grade 1 hypertension is defined as systolic or diastolic BP from 95 percentile to 99 percentile, +5 mmHg for age and height for individual gender. Grade 2 hypertension is defined as BP levels that are more than 5 mmHg above 99 percentile for age and height for individual gender. We staged severity of acute kidney injury according to RIFLE criteria. The clinical presentation and laboratory evaluation of the patients is shown in . The TTP was diagnosed clinically, by laboratory parameters and on renal biopsy. The diagnosis of TTP was secured after an expert opinion from clinical hematologist. The diagnosis of PRES was established on neuroimaging. Diagnostic workup and therapy was at the discretion of attending doctor. The nature of this study was only observational and did not demand any deviation from the standard care. So no informal consent was found necessary. The outcome was defined by clinical and radiological improvement or death.
Table 1. Clinical and laboratory data of our study.
Results
Headache was present in 100% of the patients where as hypertension was present in 87.5% of the children. The hypertension in all the patients was first time detected. Two children (case 1 and 3) had bleeding manifestation in the form of bloody diarrhea and hematuria. Case no 1 was a 5-year old boy with no significant past history presented with acute onset hypertension, headache, seizures and loose motions containing blood and mucous. His clinical presentation is shown in the . MRI of the brain showed hyperintense lesions in parieto-occipital regions and was suggestive of PRES (). In view of renal failure and proteinuria, renal biopsy was performed which revealed features of TTP in the form of significant loss of mesangial matrix, with a fluffy appearing remaining mesangial area and retraction of capillary tufts (mesangiolysis). The glomerular capillaries were congested and dilated (H&E × 250) ().
Figure 1. MRI at presentation of PRES. T2-weighted image (A1) and FLAIR image (B1) show high signal abnormalities bilaterally in the parietooccipital regions, in both subcortical white matter and cortical gray matter. MRI after 2 weeks of follow-up shows complete disappearance of all high signal abnormalities in both T2-weighted image (A2) and FLAIR image (B2).
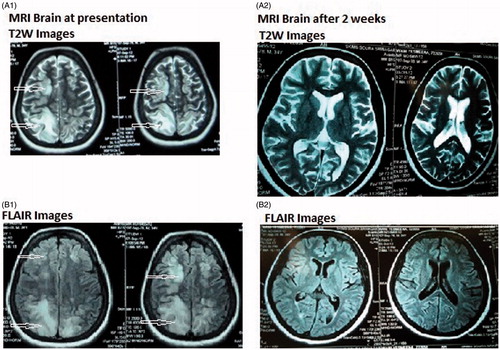
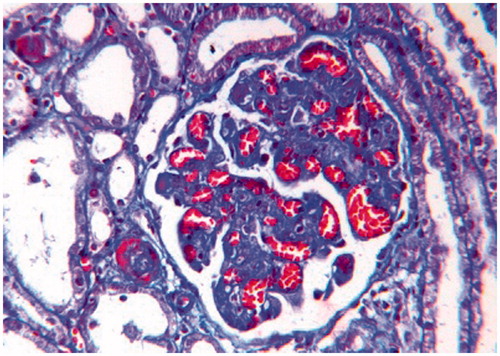
Figure 2. Glomerulus from a H & E stained section, showing significant loss of mesangial matrix, with a fluffy appearing remaining mesangial area and retraction of capillary tufts (mensagiolysis). The glomerular capillaries are congested and dilated (H&E × 250).
Case no 2 was 8-year old boy who presented with generalized tonic clonic seizures (GTCS) and encephalopathy. Patient had high blood pressure, mild renal failure, and urine microscopy showed active urinary sediment. Antistreptolysin O antibody titers were high (>600). Patient gave history of upper respiratory tract infections two weeks before. Imaging studies showed hyperintense lesions in parieto-occipital and cerebellar regions. In view of acute renal failure and urine picture, renal biopsy was performed which revealed necrosis of hilar arteriole with presence of fragmented RBC's in the wall and luminal occlusion (H&E × 250).
Case no 3 was a 14-year old female who presented with headache, altered sensorium, and hematochezia. Besides having renal failure and thrombocytopenia, urine microscopy showed proteinuria and microscopic hematuria. MRI brain was suggestive of PRES (). Other clinical and lab characteristics are shown in . Renal biopsy revealed fragmented RBC’S in the wall with congested capillary lumen and luminal occlusion suggestive of TTP ().
Figure 3. (A) Non-contrast CT head at presentation showing diffuse white matter and gray matter edema. (B) MRI brain of the same patient showing hyperintense lesions in parietooccipital area and cerebellar regions predominantly on the left side on T2W images.
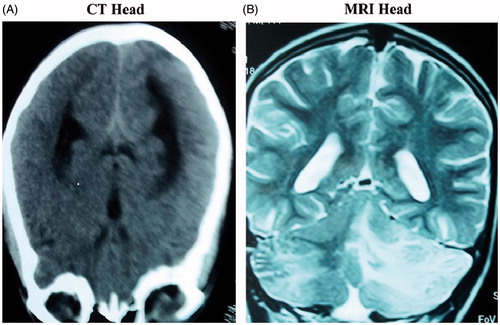
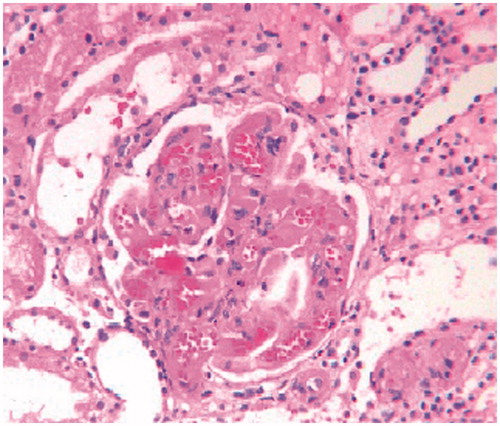
Figure 4. Photomicrographs showing ischemic shrinkage of capillary tufts with dilated and congested capillary lumina, loss of mesangial anchorage and presence of few fibrin thrombi in glomerular capillaries. (Masson's Trichrome × 250).
The clinical presentation, biochemical profile and outcome of other patients are shown in .
Laboratory evaluation
The detailed laboratory evaluation is shown in . Acute renal failure and thrombocytopenia was present in 100% of the patients. Two patients had total leukocyte count in the upper range of normal and two patients had leukocytosis. All the patients had MRI documented PRES. Follow-up MRI in three patients was normal.
Management and outcome
All the patients were managed in ICU settings. Intensive management was pursued for control of hypertension which included nitroglycerine infusion, intravenous (I.V) labetalol (SOS) and oral antihypertensive agents. Two patients (case no 2 and 5) received IV diuretics in addition. All the patients received plasma transfusions.
All the patients were followed clinically and biochemically during the period of admission. The outcome was noted in the form of recovery, death or clinical sequela. The adequate response was defined when neurologic signs and symptoms disappear, the platelet count climbs to greater than 50,000/µL, and the LDH declines. Case no 2 and 3 received 12 and 14 exchanges of plasmapheresis respectively in addition to corticosteroids and improved. Despite good control of blood pressure and supportive care, case no 1, 5 and 6 did not show any improvement and died after 5 exchanges. Case no 7 showed improvement with conservative management only whereas case no 4 died without receiving exchanges.
Among these TTP patients admitted with PRES, 57.1% of the patients died. Both the patients with bleeding manifestations died. None of the treatment responders had relapse till date.
Discussion
Although, described in adults, the occurrence of PRES in TTP pediatric patients is not described. The present study attracts novelty and uniqueness due to the following facts: (1) the clinical course and outcome of children who present with TTP and PRES has not been observed and studied till date (2) this is the first ever study in pediatric population where association of TTP and PRES has been described and studied (3) Results from our observation invite potential insight for further research on this subject.
Burrus et al. did a retrospective analysis of 47 patients having TTP. All the patients were adults more than 18 years of age. Among these patients, 13 patients had PRES. The occurrence of PRES in these patients was strongly associated with renal failure.Citation2 Gera et al. recently published a case series of 11 pediatric patients having PRES and kidney disease. The association of PRES with different risk factors was studied. None of the patients had TTP.Citation11 PRES in pediatric patients carries good prognosis if treated early. Incecik et al.Citation12 in their retrospective analysis of nine children who had PRES found that all the patients improved clinically and radiologically. de Laat et al.Citation13 described seven childhood cancer patients who had clinical and radiological evidence of PRES. About 86% of the patients had both clinical and radiological reversible symptoms. None of the patients in both the series had evidence of TTP.
In the present study, all the patients had renal failure. We found that, 57.1% children died even after best possible supportive care. The presence of PRES in pediatric population with TTP therefore increases mortality and outcome is worse. After the advent of plasmapheresis, the prognosis of TTP patients without PRES is good and more than 80% of the patients survive with supportive and conservative management.Citation1
presents comparative data from different pediatric studies including ours. Two other studies present data on PRES and TTP each. All the seven children in de Laat et al.’s study had underlying malignancy.Citation13 Hypertension and neurological signs were present in 100% of the patients. All the patients improved. In Horton et al.’s study, all the patients had TTP without PRES. 42.8% patients had bleeding manifestations, 71.4% children had neurological abnormalities and 28.5% patients had renal failure. All the seven patients improved after appropriate management.Citation14 In our study, hypertension, renal failure and neurological abnormalities were present in 100% of the patients where as bleeding manifestations through gastrointestinal and urinary tract were present in 28.5% of the patients. Thrombocytopenia was present in 71.4% of the patients. McDonald et al. reported seven children who had non congenital TTP. All cases had ADAMTS13 activity less than 5% and an inhibitor to ADAMTS13. All cases improved with conservative management.Citation15 It is clear from these observations that, either of the two conditions when present alone show favorable outcome. The clinical course worsens and outcome is poor when two conditions are associated. The factors that predispose patients with TTP to PRES are not known. The occurrence of PRES in TTP patients has been related to severity of renal failure.Citation2
Table 2. Comparison between present study and two other pediatric studies.
The more common causes of PRES include hypertensive emergencies, pre-eclampsia and treatment with immunosuppressive drugs. Less common causes include uremia, digitoxin toxicity and dialysis disequilibrium.Citation6,Citation16 TTP as a cause of PRES is not commonly described. The effect of PRES on the clinical course of TTP in pediatric patients has not been studied. According to our observation, TTP which otherwise carries good prognosis is complicated by the presence of PRES and outcome is not favorable even after plasmapheresis. The occurrence of PRES in TTP patients unrelated to hypertension has been shown in TTP cohort described by Burrus et al.Citation2 The underlying pathophysiological mechanisms for PRES in TTP patients remain elusive. Horbinski et al.Citation17 confirmed histologically the endothelial activation and the presence of markers of cell-mediated immune response in a case described by them as having PRES. Also, PRES has been described in patients with neuromyelitis optica and aquaporin 4 autoimmunity.Citation18 Aquaporin 4 is a component of the astrocytic foot process in the blood-brain barrier. It acts as a well-known water conduit that, when altered, leads to vasogenic edema. The favored pathogenic theory for PRES suggests autoregulatory disturbance with hyperperfusion, resulting in the shifting of fluid from the intravascular compartment and consequent vasogenic edema.Citation5 It is unknown why the posterior circulation is preferentially affected. A possible explanation is the lower sympathetic innervation of posterior cerebral arterial circulation, with a consequent reduced autoregulation of already impaired cerebral areas.Citation19
Our study is limited by small number of patients. Furthermore, all the patients were managed in ICU. Increased mortality being related to severity of other abnormal clinical and laboratory parameters could not be analyzed statistically because our cohort of patients was small. Therefore further studies which include large number of patients and which are observational in nature are needed to study the association.
The reversibility of the PRES was confirmed both clinically as well as by neuroimaging studies in three cases. The occurrence of PRES in our cohort of patients could be attributed to accelerated hypertension, uremia or TTP per se.
Conclusion
TTP carries favorable prognosis in adults. In children, prognosis is good if treatment is started early. According to our observation, the clinical course in TTP pediatric patients is complicated and with PRES, the outcome is not good. Our observation should be taken with some limitations. The sample size in our study is small. The effect of other associated factors like severity of illness and presence of other clinical and laboratory abnormalities cannot be ruled out. This observation potentially encourages researchers to study the subject further and better so that this presumed potentially dangerous association is explored further.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Meloni G, Proia A, Antonini G, et al. Thrombotic thrombocytopenic purpura: Prospective neurologic, neuroimaging and neurophysiologic evaluation. Hematologica. 2001;86:1194–1199
- Burrus TM, Wijdicks EF, Rabinstein AA. Brains lesions are most often reversible in acute thrombotic thrombocytopenic purpura. Neurology. 2009;73:66–70
- Burrus TM, Mandrekar J, Wijdicks EF, Rabinstein AA. Renal failure and posterior reversible encephalopathy syndrome in patients with thrombotic thrombocytopenic purpura. Arch Neurol. 2010;67:831–834
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior encephalopathy syndrome. N Engl J Med. 1996;334:494–500
- Kwon S, Jahoon K, Sangkwon L. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol. 2001;24:361–364
- Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: Prognostic utility of quantitative diffusion-weighted MR images. Am J Neuro Radiol. 2002;23:1038–1048
- Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: Utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. Am J Neuro Radiol. 2000;21:1199–1206
- Servillo G, Striano P, Striano S, et al. Posterior reversible encephalopathy syndrome (PRES) in obstetric critically ill patients. Intensive Care Med. 2003;29:2323–2326
- Sheth KN, Wu GF, Messe SR, et al. Dialysis disequilibrium: Another reversible posterior leukoencephalopathy syndrome? Clin Neurol Neurosurg. 2003;105:249–252
- The fourth report on the diagnosis, evaluation, treatment of high blood pressure in children and adolescents. National high blood pressure education program working group on high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576
- Gera DN, Patil SB, Iyer A, et al. Posterior reversible encephalopathy syndrome in children with kidney disease. Indian J Nephrol. 2014;24:28–34
- Incecik F, Hergüner MO, Altunbasak S, Erbey F, Leblebisatan G. Evaluation of nine children with reversible posterior encephalopathy syndrome. Neurol India. 2009;57:475–478
- de Laat P, Te Winkel ML, Devos AS, Catsman-Berrevoets CE, Pieters R, van den Heuvel-Eibrink MM. Posterior reversible encephalopathy syndrome in childhood cancer. Ann Oncol. 2011;22:472–478
- Horton TM, Stone JD, Yee D, Dreyer Z, Moake JL, Mahoney DH. Case series of thrombotic thrombocytopenic purpura in children and adolescents. J Pediatr Hematol Oncol. 2003;25:336–339
- McDonald V, Liesner R, Grainger J, Gattens M, Machin SJ, Scully M. Acquired, noncongenital thrombotic thrombocytopenic purpura in children and adolescents: Clinical management and the use of ADAMTS 13 assays. Blood Coagul Fibrinolysis. 2010;21:245–250
- Kwon S, Jahoon K, Sangkwon L. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol. 2001;24:361–364
- Horbinski C, Bartynski WS, Carson-Walter E, Hamilton RL, Tan HP, Cheng S. Reversible encephalopathy after cardiac transplantation: Histologic evidence of endothelial activation, T-cell specific trafficking, and vascular endothelial growth factor expression. AJNR Am J Neuroradiol. 2009;30:588–590
- Magana SM, Matiello M, Pittock SJ, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72:712–717
- Schwartz RB, Feske SK, Polak JF, et al. Preeclampsia-eclampsia: Clinical and neuroradiographic correlates and insight into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371–376

