Abstract
Sleep disorders are common among the patients undergoing dialysis in end stage renal disease (ESRD). Although variable, their prevalence has been reported to be higher when compared to the general population. The most frequently reported complaints are insomnia, restless leg syndrome (RLS), sleep-disordered breathing and excessive daytime sleepiness (EDS). The aim of this study was to assess the prevalence of sleep disorders in end stage renal disease patients on regular hemodialysis (group I with 30 patients) and CKD patients (group II with 30 patients) in comparison to 30 normal population (control group). In addition to laboratory investigations which included creatinine clearance using Cockroft and Gault formula, hemoglobin level (Hb), blood urea, serum creatinine, serum albumin, serum calcium and phosphorus and lipid profile, all subjects underwent one night of laboratory-based polysomnography (PSG) consisting of a standard montage of electroencephalography (EEG) (C3/A1 and O2/C3 or O1/C4), monopolar left and right electrooculography (EOG) referenced to the opposite mastoid, surface mentalis electromyography (EMG), respiratory airflow (measured by thermistor) and effort (piezoelectric sensors), electrocardiography (ECG), anterior tibialis EMG and pulse oximetry. For hemodialysis subjects, this study was performed on a night immediately following hemodialysis treatment. The results showed that patients on hemodialysis have sleep disorders, and that sleep disorders are common in group I and II than control group. The percentage of sleep disorders in hemodialysis patients were as follows: insomnia (69%), followed by obstructive sleep apnea syndrome OSAS (24%), RLS and periodic limb movement PLM (18%), nightmares (13%), EDS (12%), sleepwalking (2%), possible rapid eye movement behavior disorders RED (2%), possible narcolepsy (1.4%). While the percentage of sleep disorders in CKD patients were as follows: insomnia (54%), followed by RLS (19%), PLM (12%), OSAS (16%), nightmares (15%), EDS (15%), sleepwalking (4%), possible RBD (3%), possible narcolepsy (1%). There was inverse correlation between sleep disorders and Hb, albumin and creatinine clearance; also there was positive correlation between sleep disorder and phosphorus. We concluded that the sleep disorders are common in CKD patients either on conservative management or on regular hemodialysis. Treatment of anemia, hyperphosphatemia and hypoalbuminemia may improve sleep disorders among those patients.
Introduction
Sleep disorders are common among patients undergoing dialysis in end stage renal disease (ESRD). Although variable, their prevalence has been reported to be higher when compared to the general population. The most frequently reported complaints are insomnia, restless leg syndrome (RLS), sleep-disordered breathing and excessive daytime sleepiness (EDS).Citation1 The prevalence of sleep apnea (SA) in ESRD and dialysis patients has been reported in (13–70%),Citation1 which is much higher than the general population (2–4%).Citation2 This large variation in the prevalence of SA in dialysis patients is probably due to different populations being studied, the method of diagnosis [whether it is based on questionnaires or polysomnography (PSG)] and the definition used to diagnose SA. Excessive daytime sleepiness (EDS), a major consequence of SA, is caused by sleep fragmentation that is triggered by repetitive episodes of partial or complete upper airway obstruction. Sleep fragmentation may also contribute to impaired cognition and altered moods as well as subject the patient to increased risk of work- or driving-related accidents.Citation3 The incidence of sleep disorders and its causes in patients undergoing hemodialysis have attracted the attention of many researchers in the past 10 years. Renal replacement therapy exposes the patients to the risk of a wide range of physical, psychological, economic, and social problems, and influences their quality of life in general.Citation4 Studies have focused on patients on maintenance hemodialysis, whereas patients with mild/moderate chronic kidney disease (CKD) have been neglected. In fact, only a few studies on this category of patients are available. Therefore, this study was devised to evaluate the prevalence of sleep disturbances in CKD patients and also hemodialysis patients and to examine the relationship of sleep disorders to renal function and to laboratory parameters.
Materials and methods
This study was conducted on 90 subjects and they were divided into three groups. Group I (hemodialysis group) included 30 patients with ESRD on regular hemodialysis three sessions weekly, four hours per session. Vascular access was achieved through either a long-term internal jugular catheter or an arteriovenous fistula. Dialysate flow rate of 500 mL/min and high flux dialyzers were used. Group II (CKD group) included 30 patients with CKD under conservative treatment, their creatinine clearance ranged from 15 to 50 mL/min. In addition to group III (control group) included 30 healthy volunteers. We excluded subjects on medication with known effects on sleep-related measures, patients with neurological disorders, patients with significant mental illness requiring psychiatric treatment (including those previously diagnosed with anxiety or depression and/or taking medication for those conditions). Also the patients with comorbidities associated with nocturnal symptoms (congestive heart failure, unstable angina, arthritis and chronic obstructive pulmonary disease) were excluded. Obese patients (BMI more than 35) and those with a history of previously diagnosed and/or treated sleep disorders were also excluded. We obtained history, clinical examination and laboratory investigations from all the patients, which included creatinine clearance using Cockroft and Gault formula,Citation5 hemoglobin level (Hb), blood urea, serum creatinine, serum albumin, serum calcium and phosphorus and lipid profile. All the subjects underwent one night of laboratory-based polysomnography (PSG) consisting of a standard montage of electroencephalography (EEG) (C3/A1 and O2/C3 or O1/C4), monopolar left and right electrooculography (EOG) referenced to the opposite mastoid, surface mentalis electromyography (EMG), respiratory airflow (measured by thermistor) and effort (piezoelectric sensors), electrocardiography (ECG), anterior tibialis EMG and pulse oximetry.Citation6 For hemodialysis subjects, this study was performed on a night immediately following hemodialysis treatment.
Statistical analysis
The statistical analysis of data was performed by using excel program and the statistical package for social science (SPSS) program version 10 (Cairo, Egypt). The description of the data done was written in the form of the mean (±) SD for quantitative data. The analysis of the data was done to test the statistically significant difference between the groups, where p value < 0.05 was considered as significant. For quantitative data, Student’s t-test was used to compare the two groups and paired sample t-test was used to compare one group at different measurements. One way analysis of variance (ANOVA) test with post hoc analysis was used to compare more than two groups. To test the association between the variables, Pearson correlation co-efficiency test was used.
Results
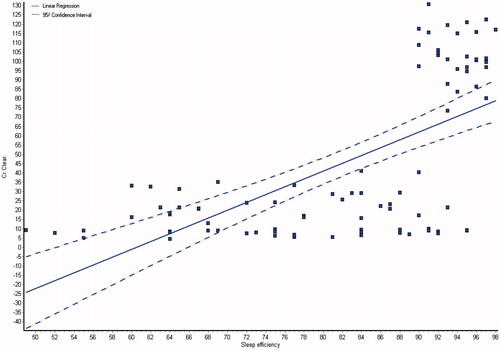
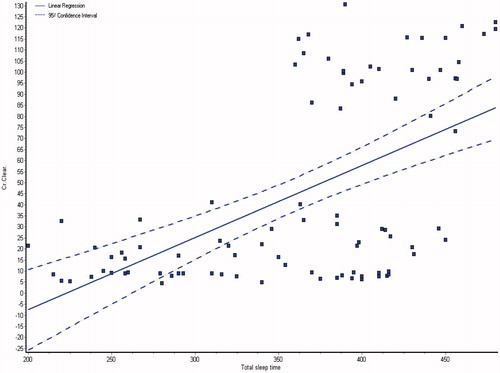
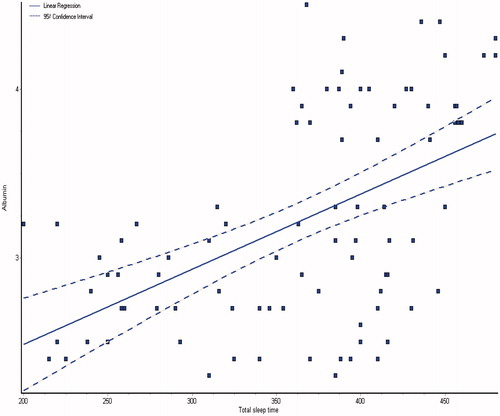
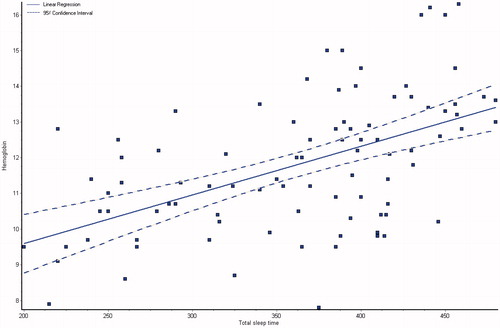
and showed the demographic and laboratory parameters for both groups I and II in addition to control group (group III). Data from the observed polysomnographic (PSG) measures for all groups are shown in . Both groups I and II had reduced total sleep time (≤6 h) in comparison with normal control subjects. However, in comparison with CKD group, the HD group had less total sleep time. The HD group had significantly less REM sleep, but there were no differences between the groups as regard to percentage of specific NREM sleep stage. There was a highly significant difference of sleep latency, sleep efficiency, wake after sleep onset, brief arousal index, periodic limb movement index and respiratory disturbance index in all the groups (p < 0.0001), they were higher in groups I and II as shown in . The total sleep time and sleep efficiency were negatively correlated with blood urea, serum creatinine and phosphorus, and positively correlated with albumin, hemoglobin and creatinine clearance as shown in ,, and . While, the brief arousal index and periodic limb movement index were negatively correlated albumin, hemoglobin and creatinine clearance, and positively correlated with blood urea, serum creatinine and phosphorus as shown in .
Table 1. Demographic features for group I and II and III.
Table 2. Laboratory characteristics for all the studied groups.
Table 3. Characteristics of polysomnographic sleep measures for the studied group.
Table 4. Correlation coefficient between total sleep time (min), sleep efficiency and periodic limb movement index with laboratory findings in all the studied groups.
Discussion
Numerous factors probably contribute to the high prevalence of sleep problem in HD patients including psychological responses, metabolic changes and treatment-related factors. In contrast the sleep of patients with chronic kidney disease not yet receiving treatment has received little attention. However, poor sleep quality is also prevalent in this group, but may be more related to psychological factors rather than metabolic changes associated with renal disease.Citation7 Therefore, the objective of this study was to compare polysomnographic measures of nocturnal sleep in stable patients on chronic, intermittent hemodialysis in comparison with an age- and gender-matched, metabolically comparable group with CKD patients. We found that both the groups had reduced total sleep time and sleep efficiency in comparison with normative data, an observation consistent with the reported complaints of poor sleep. Other than those similarities, the sleep features of the two group differed. The CKD group had normal average sleep latency, while that of the HD group was almost twice as long. We also found that significantly less REM sleep and increased numbers of brief arousals among the hemodialysis patients, also they had more frequent apneas and limb movements. Those finding suggest that the mechanisms underlying the poor sleep reported by patients on chronic, intermittent daytime HD and CKD may differ. Despite elimination of potential subjects with significant physical and psychological morbidities in both the groups, CKD subjects reported poorer quality of life especially with regard to functional and psychological status. It may be the prospect of disease progression and eventual need for the treatment may affect selected measures of sleep such as total sleep time. In contrast the HD group may have reported higher quality of life because of the opportunity to adjust to both the illness and treatment. The greater number of brief arousal, apneas and limb movement noted in HD group suggests that the chronic, intermittent daytime form of this treatment may have iatrogenic effects on sleep. This observation is consistent with the finding reported by other studyCitation8 which found that the administration of slow, nightly HD significantly decreased apnea “although it did not decrease arousals and limb movements”, possible due to enhancement of ventilator stability. Collectively, those results suggest that administration of the treatment in a more stable, consistent manner, and/or having stable residual renal function may have beneficial effects on sleep. Treatment time of the day may also be a contributing factor. In contrast, there are several mechanisms via which chronic, intermittent daytime HD (induce very rapid physiological changes) may alter biological systems controlling sleep stability and quality. For example, this type of treatment has been associated with increased daytime sleep that can decrease the quantity and quality of subsequent nocturnal sleep. The rapid fluid, electrolyte and acid/base changes that occur are often associated with central nervous system symptoms such as changes in arousal and fatigue during or immediately after treatment. A fall in cerebral spinal fluid PH during dialysis and slow movement of bicarbonate across the blood brain barrier may also be contributing factors causing daytime somnolence and subsequent decreased nocturnal sleep quality and ventilator instability.Citation7 Under-dialysis was considered by Chen and colleagues as a factor that increased the likelihood of sleep disruption in hemodialysis patients,Citation2 while Perl and associates regarded the sleep abnormality as a marker of inadequate dialysis.Citation9 In a study conducted by Christopher and his colleagues, they found that by augmenting dialysis dose and frequency by nocturnal hemodialysis (eight to ten hours, six nights per week), they corrected night-time hypoxemia, lowered heart rate, and returned the autonomic modulation of heart rate variability during sleep to a more normal state of vagal-sympathetic balance.Citation10 Also Rajiv and Robert concluded in their study that missing dialysis was associated with greater burden of sleep disturbance.Citation11 In our study the percentage of sleep disorders in hemodialysis patients were as follows: insomnia (69%), followed by OSAS (24%), RLS and PLM (18%), nightmares (13%), EDS (12%), sleepwalking (2%), possible RBD (2%), and possible narcolepsy (1.4%). While the percentage of sleep disorders in CKD patients were as follows: insomnia (54%), followed by RLS (19%), PLM (12%), OSAS (16%), Nightmares (15%), EDS (15%), sleepwalking (4%), possible RBD (3%), and possible narcolepsy (1%). Our results showed positive correlation between albumin level and sleep efficiency and total sleep time in all the studied groups (r = 0.579, p < 0.0001 and r = 0.5234, p < 0.0001), respectively. A negative correlation was found between albumin level and brief arousal index and PLM in all the studied groups (r = −0.6607, p < 0.0001 and r = −0.581, p < 0.0001), respectively. These results were in agreement with the results of other investigatorsCitation12,Citation13 who found that hypoalbuminemia reported to be associated with disturbed sleep insomnia and RLS. In our study there was positive correlation between Hb level and sleep efficiency and total sleep time in all the studied groups (r = 0.371, p = 0.0003 and r = 0.531, p < 0.0001) respectively, but a negative correlation was found between Hb level and brief arousal index and PLM (r = −0.543, p < 0.0001 and r = −0.5087, p < 0.0001), respectively. These results were in agreement with many observations reported by the other investigators.Citation7,Citation13,Citation14 As regard to phosphate level and sleep disorders there was positive correlation between phosphate level and brief arousal index and PLM in all the groups (r = 0.367, p = 0.0004 and r = 0.384, p = 0.0002), respectively, While negative correlation was found between phosphate level and sleep efficiency in all groups (r = −0.4131, p < 0.0001 and r = −0.3987, p < 0.0001), respectively. On the other hand, sleep disorders were also reported in many cases with chronic illness such as liver cirrhosis and heart failure. Sleep-related complaints and disturbances are increasingly recognized in the setting of chronic liver disease and have recently been shown to be an important prognostic factor in patients with advanced chronic liver disease.Citation15 In a study conducted by Gencdal and others on 131 cirrhotic patients and 18 healthy volunteers, they found that sleep disorders were common in cirrhotic patients, most in Child C patients.Citation16 Also, Vinicius et al., studied sleep aspects and parameters in 42 cirrhotic patients, and 42 controls and assessed the role of liver dysfunction severity in polysomnographic results. They concluded that cirrhosis was associated with shorter sleep time, reduced sleep efficiency, increased sleep latency, increased REM latency and reduced REM sleep. Additionally, disease severity influences sleep parameters.Citation17 These findings similar to what we encountered in CKD and hemodialysis patients. Heart failure (HF) is also associated with high levels of sleep disturbance and sleep disorders, including insomnia, periodic limb movements during sleep, and sleep disordered breathing.Citation18 One study was conducted by Johansson et al., on 331 elderly, to compare the prevalence of sleep disordered breathing and insomnia between elderly with heart failure and age and gender matched elderly without cardiovascular disease. They concluded that sleep disordered breathing, difficulties maintaining sleep and excessive daytime sleepiness were more common in elderly with heart failure.Citation19
Conclusion
We conclude that the Sleep disorders are common in CKD patients either on conservative management or on regular hemodialysis. Treatment of anemia, hyperphosphatemia and hypoalbuminemia may improve sleep disorders among those patients. Future expansion of this work by using overnight slow low-efficient dialysis sessions for the patients with sleep disorders may be provided a proper therapy in this category of patients. Other chronic systemic diseases like liver cirrhosis and heart failure are one of the most important factors that can affect the characteristics of patents sleep for a long-time period. Chronic diseases cause various sleep problems and impair sleep quality.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Merlino G, Piani A, Dolso P, et al. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant. 2006;21:184–190
- Chen WC, Lim PS, Wu WC, et al. Sleep behavior disorders in a large cohort of Chinese (Taiwanese) patients maintained by long-term hemodialysis. Am J Kidney Dis. 2006;48(2):277–284
- Mahowald MW, Bornemann MA. Sleep and ESRD: A wake-up call. Am J Kidney Dis. 2006;48(2):332–334
- Parvan K, Lakdizaji S, Roshangar F, Mostofi M. Quality of sleep and its relationship to quality of life in hemodialysis patients. J Caring Sci. 2013;2(4):295–304
- Cockcroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41
- Sivan Y. Normal polysomnography in children and adolescents. Chest. 2005;127(3):1080
- Iliescu EA, Coo H, McMuurray MH, et al. Quality of sleep and health-related quality of life in hemodialysis patients. Nephrol Dial Transplant. 2003;18(1):126–132
- Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102–107
- Perl J, Unruh ML, Chan CT. Sleep disorders in end-stage renal disease: Markers of inadequate dialysis? Kidney Int. 2006;70(10):1687–1693
- Chan CT, Hanly P, Gabor J, Picton P, Pierratos A, Floras JS. Impact of nocturnal hemodialysis on the variability of heart rate and duration of hypoxemia during sleep. Kidney Int. 2004;65:661–665
- Agarwal R, Light RP. Sleep and activity in chronic kidney disease: A longitudinal study. Clin J Am Soc Nephrol. 2011;6:1258–1265
- Bilgic A, Akugl A, Sezer S, Arat Z, Ozdemir FN, Haberal M. Nutritional status and depression, sleep disorders, and quality of life in hemodialysis patients. J Ren Nutr. 2007;17:381–388
- Alaa S, Hamdy A, Ehab W, et al. Sleep disorders in hemodialysis patients. Saudi J Kid Dis Transpl. 2010;21(2):300–305
- Pai MF, Hsu SP, Yang SY, Ho TI, Lai CF, Peng YS. Sleep disturbance in chronic hemodialysis patients: The impact of depression and anemia. Ren Fail. 2007;29(6):673–677
- De Cruz S, Espiritu JR, Zeidler M, Wang TS. Sleep disorders in chronic liver disease. Semin Respir Crit Care Med. 2012;33(1):26–35
- Gencdal G, Gunsar F, Meral CE, et al. Sleep disorders in cirrhotics; how can we detect? Liver Int. 2014;34(8):1192–1197
- Teodoro VV, Bragagnolo MA Jr, Lucchesi LM, et al. Polysomnographic sleep aspects in liver cirrhosis: A case control study. World J Gastroenterol. 2013;19(22):3433–3438
- Redeker NS. Sleep disturbance in people with heart failure: Implications for self-care. J Cardiovasc Nurs. 2008;23(3):231–238
- Johansson P, Arestedt K, Alehagen U, Svanborg E, Dahlström U, Broström A. Sleep disordered breathing, insomnia, and health related quality of life – A comparison between age and gender matched elderly with heart failure or without cardiovascular disease. Eur J Cardiovasc Nurs. 2010;9(2):108–117