Abstract
Renal ischemia reperfusion injury (IRI) contributes to the development of acute kidney injury (AKI). Several processes are involved in the development of renal IRI with the generation of reactive oxygen species, inflammation and apoptosis. MicroRNAs (miRNAs) are endogenous, small and noncoding RNAs that repress gene expression of target mRNA in animals post-transcriptionally. miRNA-mediated gene repression is a major modulatory mechanism to regulate fundamental cellular processes such as the cell cycle, proliferation, growth, and apoptosis, which in turn have pivotal influences on pathophysiological outcomes. Recent studies have revealed the pathogenic roles played by miRNAs in many renal diseases, such as IRI, AKI and renal carcinoma. In addition, the majority of miRNAs identified appear to be differentially expressed, probably to quell the injury response by modulating inflammation, apoptosis and proliferation and may point us toward new pathways that can be targeted to regulate or prevent renal IRI. They may represent novel diagnostic biomarkers of renal IR injury.
Introduction
Ischemia (cessation of blood flow), followed by reperfusion (re-establishment of blood flow), causes injury to tissues. Reperfusion is essential for preventing ischemic cell death. However, reperfusion contributes to cellular injury—a phenomenon known as reperfusion injury—which is associated with the return of oxygen. Therefore, ischemic injury is precipitated by a lack of oxygen, while reperfusion injury is associated with the return of oxygen. Thus, ischemia reperfusion (IR) contributes to major tissue dysfunction associated with many diseases.Citation1
Ischemia reperfusion injury (IRI) results in the clinical syndrome of acute kidney injury (AKI), a common clinical problem. The initial hypoxic injury and reperfusion leads to activation of immune responses, resulting in tissue damage. After injury, a repair process involving cellular proliferation must occur to regain kidney function. Also, IRI is inevitable in renal transplantation and contributes to renal transplant dysfunction and changes in renal transplants that affect outcome. However, the pathogenesis of renal IRI is not fully understood.Citation2
IRI in tissues and organs leads to the progress of ischemic diseases, including stroke in the brain and myocardial infarction. In kidneys, IRI is a main cause of acute renal failure (ARF). The pathogenesis of IRI is complex and involves a marked inflammatory response.Citation3 It stimulates renal proximal tubules to increase production of pro-inflammatory cytokines and chemokines. Also, inflammation can take place with ARF and a recruitment of circulating inflammatory cells may occur. Thus, with renal cytokine efflux into the systemic circulation, external tissue injury may result.Citation4
It has been recognized that the loss of functioning tubular epithelial cells in kidney IRI is caused by both necrosis and apoptosis. In the kidney, Bcl2 is a major cell survival or development proto-oncogene. In fetal renal development, the distribution of apoptotic cells is inversely correlated with Bcl2 expression. In renal IRI, Bcl2 expression highly increases in the distal tubules and is associated with increased survival of both the distal and proximal segments at acute phases. After renal injury, Bcl2 expression is increased in regenerating proximal tubule cells.Citation5
Reactive oxygen species in renal IRI
Reactive oxygen species (ROS) play an important role in the pathology of renal IRI. ROS production has been implicated in necrosis and apoptotic cell death in IRI. Oxidative stress causes lipid peroxidation and ATP depletion.Citation6 High levels of ROS can oxidize many cell constituents, including proteins, DNA and lipids, and impose a threat to cell integrity. Cells have evolved many defense mechanisms to cope with oxidative stress, among which autophagy plays a main role. There is accumulating evidence for specific autophagic processes in response to ROS. These include: (1) chaperone-mediated autophagy, proposed to exhibit higher efficiency in degrading oxidized proteins than their unaltered counterparts, (2) autophagy in plants, demonstrated to act in the degradation of oxidized proteins after sever oxidative stress, and (3) the degradation of mitochondria, termed mitophagy, suggested to decrease the potential oxidative damage caused by defective mitochondria. ROS are important both in protein aggregate formation and in autophagy.Citation7 Members of the small heat shock protein (HSP) family (the HSP20 family), which includes HSP25/27 are molecular chaperones involved in increasing tolerance to cytotoxic stresses, also, they may possess anti-apoptotic functions.Citation8 ROS play an important role in the pathogenesis of renal failure and renal IRI. ROS such as hydrogen peroxide (H2O2) and superoxide-elicited expression changes of multiple genes are responsible for ROS-mediated cell injury responses such as necrosis and apoptosis.Citation9
MicroRNAs
MicroRNAs (miRNAs) are recently discovered endogenous, small, single stranded noncoding RNAs of approximately 22 nucleotides. They post-transcriptionally modulate gene expression by hybridization to messenger RNA (mRNA), leading to translational degradation or repression of the target mRNA. The mature miRNAs bind to the 3′ untranslated region (UTR) of their mRNA targets and negatively regulate gene expression via degradation or translational inhibition. MiRNAs have emerged as one of the central players of gene expression modulation.Citation10,Citation11
About 700 miRNAs have been identified in humans. As a group, miRNA may regulate at least 30% of the genes in a cell. Estimates demonstrate that miRNAs may modulate up to one-third of the mammalian genome. Although a large number of miRNAs have been discovered only a few target genes have been identified and the functions of most of them remain unknown. Also, they may be involved in the regulation of almost all cellular functions, such as cell growth, differentiation, mobility and death (necrosis and apoptosis). Thus, they could be the pivotal regulators in physiology, disease and development. Although miRNAs characterize new gene expression regulators at the translational level, the effects of ROS on miRNA expression and the roles of miRNAs in ROS-mediated gene regulation are unclear. The expression changes of miRNAs after ROS stimulation could be important in ROS-mediated modulations of signaling transduction pathways and gene expression.Citation9 Dys-regulated miRNA expression has been reported to be involved in the brain and heart IRI. However, the endogenously synthesized miRNAs have been indicated to be protective following IR injury.Citation10
Cell- and tissue-specific expressions are major characteristics of miRNA expression. Indeed, one miRNA may be highly expressed in one cell or tissue, but has no or low expression in other cells or tissues. For example, miR-1 is reported to be a heart-specific miRNA, whereas miR-145 is a vascular smooth muscle cell-specific miRNA. The tissue-specific miRNA expression and tissue expression signatures of diseases have provided a major diagnostic opportunity for different diseases. The studies have recently revealed that miRNAs exist in circulating blood. In addition, cancer tissue miRNAs are able to be released into circulating blood and serum can be used as novel biomarkers for different cancers. Therefore, a quantitative method to measure the amount of a miRNA in blood has been established. For example, a quantitative method to measure the serum level of miR-1 was established based on quantitative real time-PCR technology.Citation12
Diverse diseases have different miRNA expression profiles. However, diseased tissues are difficult to obtain under disease conditions. But, miRNAs are able to be released into circulating blood from tissues. Recent reports have revealed that circulating miRNAs released from tissues are stable due to binding with other materials.Citation12 Thus, circulating cell-free miRNAs can be used as novel biomarkers for diverse cancers. miRNAs in the peripheral blood have been proven to be useful biomarkers for diseases such as liver injury and cancer.Citation12
miRNAs in renal IR injury
miRNAs, by regulating gene expression, play important roles in different cellular and physiological activities.Citation13 miRNA expression is frequently altered, in human diseases, contributing to pathogenesis.Citation14 The role of miRNAs in the modulation of renal physiology and pathology has emerged as an important area of research.Citation15 The studies on the miRNAs in diverse renal diseases may lead to emerging of new diagnostic tools and therapeutic interventions. In this review, we aim to discuss the roles of miRNAs in renal IR injury and identify research directions in this field. A role for miRNAs in renal disease is rapidly emerging; several hallmarks of IRI, such as fibrosis, apoptosis, epithelial mesenchymal transition and Toll-like receptor (TLR) signaling are regulated by miRNAs in other settings. Also, recent studies have indicated a role for miRNAs in the regulation of hepatic and cardiac IRI. It is hypothesized that miRNA expression patterns may act as a biomarker of renal injury.Citation16
Recent studies propose that miRNAs function in various cellular processes, such as cell differentiation, metabolism and apoptosis. Researches show that miRNAs cause protection against renal IRI, apparently through mechanisms involving up-regulating anti-apoptotic genes and repression of apoptotic genes. Many researchers have identified some miRNAs that are involved in renal disease. According to previous studies, the expression profile of miRNAs appears to be tissue specific.Citation17 There is evidence for the potential role of endogenously synthesized miRNAs in renoprotection following IR injury. miRNAs have many advantages over other exogenous agents. For example, they are natural cellular products therefore, nontoxic to cells. MiRNAs can be induced in vivo under natural conditions, such as hyperthermia. Due to their short length, they can easily move across and around sub-cellular structures. Thus, identifying the role of endogenously synthesized miRNAs in protective pathophysiological stimuli including heat shock, ischemic and by pharmacological means may open up novel strategies to protect the kidney in patients with renal failure.Citation17
miRNAs in AKI
Renal IR is a common problem during renal transplantation, leading to renal dysfunction and AKI. AKI is associated with high mortality rates with few effective treatments. The discovery of miRNAs as pivotal modulators of cell activities proposes the involvement of miRNAs in the pathogenesis of diverse renal diseases, such as chronic and acute renal diseases. AKI is associated with increased risk of chronic kidney disease (CKD).Citation18 Despite decades of investigation, novel and effective therapeutic approaches for AKI are still lacking. Recent findings have discovered the role of miRNAs in AKI. These studies raise hopes for effective diagnostic and therapeutic strategies.
The first finding for a pathogenic role of miRNAs in AKI was indicated by using a conditional Dicer-knockout model, in which Dicer was ablated specifically from renal proximal tubular cells.Citation3 Dicer is the enzyme responsible for the processing of pre-miRNAs into mature, functional miRNAs. Genetic ablation of Dicer leads to global depletion of miRNAs.Citation19 Mice in this model demonstrate normal renal development, and function. However, when challenged by renal IR, the conditional Dicer-null mice are resistant to the ensuing AKI compared with their wild-type littermates.Citation3 Better renal function significantly improved animal survival of Dicer-deficient mice provides compelling evidence for a pathogenic role of Dicer and associated miRNAs in ischemic AKI.
The microarray analysis has showed significant expression changes in multiple miRNAs after renal IR injury. Notably, while some miRNAs are induced, others are down-regulated in damaged tissues. However, while some miRNAs change only at one time point, other miRNAs indicate a continuous change during reperfusion.Citation3 Also, Godwin et al.,Citation16 by miRNA microarray, showed miRNA expression changes during renal IR in C57BL/6 mice. However, the miRNAs species (e.g., miRNA-132, -362, -379, -668 and 687) that indicate significant changes in these studiesCitation3,Citation16 do not overlap. By analysis of specific miRNAs and profiling of miRNA expression, future researches are expected to indicate miRNA changes in different models of IRI, resulting in the identification of potential targets for therapeutic intervention.
miRNAs as diagnostic tools in renal IR injury
In recent years, there is a major thrust to identify novel biomarkers for renal IR. Renal IR contributes to the development of ischemic AKI.Citation20 miRNA studies in diverse renal diseases have demonstrated not only that miRNA expression is differentially modulated but also that the expression pattern itself could be an effective tool for disease diagnosis. The changes of miRNAs in renal IR, tissue-specific expression patterns, methods of miRNA analysis, and their presence in urine and blood make miRNAs ideal candidates as renal disease biomarkers.Citation21,Citation22
Microarray analysis needs to be repeated with samples from separate experiments for better identification of miRNA expression. In addition, the miRNAs identified from the microarray analysis to the study need to be subjected to further verification individually by real-time PCR analysis. Also, the real-time PCR technique used for miRNA detection is not well standardized, therefore it is useful to confirm the real-time PCR results using other techniques like Northern blot analysis.Citation23 Thus, one of the most areas of emphasis in future investigations is identifying the functional significance of individual miRNAs in specific kidney diseases.
MicroRNA-1
MiRNA-1 (miR-1), among the known miRNAs, is believed to be expressed in skeletal and cardiac muscle tissues. It can modulate myogenesis by controlling distinct aspects of the differentiation process. MiR-1 had been suggested to be involved in modulating apoptosis. The miR-1 level increased in response to oxidative stress and this increase decreased the levels of two anti-apoptotic molecules, HSP60 and HSP70, without changing their transcript levels. Apoptosis plays a crucial role in IRI and the apoptosis rate decreases in miR-1 overexpression cells. There is interaction between miR-1 and Bcl2.Citation11 Bcl2 is an important regulator of apoptosis, and it is a cell death inhibitor. There are many apoptosis modulating mechanisms, which are associated with Bcl2. Recent studies have verified that repression of Bcl2 by miR-1 is probably one of the mechanisms underlying their modulation of apoptosis versus survival. Studies revealed pathological elevations of miR-1 levels in conditions favoring apoptosis (oxidative stress and ischemia reperfusion). These findings could help us better understand IR pathology and promote effective treatments.Citation11
MiR-1 is the most abundant miRNA in heart and it is a heart-specific miRNA. Cardiac tissue miR-1 is associated in the pathogenesis of cardiac diseases. The trace amount of miR-1 released into the circulating blood under physiological conditions may be responsible for the low basal serum level of miR-1. However, after acute myocardial infarct (AMI), serum miR-1 levels were rapidly increased. These findings suggest that serum miR-1 is a novel sensitive biomarker for AMI. Using circulating cell-free miRNAs as diagnostic biomarkers may represent a new revolution in heart diseases.Citation12
MicroRNA-21
MiR-21 is found to be an anti-apoptotic miRNA, although its anti-apoptotic effect is cell specific. For example, miR-21 has a strong anti-apoptotic effect on cancer cells. Also, miR-21 has an apoptotic effect on cardiac cells induced by hydrogen peroxide.Citation24 The studies identify induction of miR-21 through both cell-intrinsic and extrinsic pathways as pivotal in preventing tubular epithelial cell apoptosis. Recent data propose that relatively few miRNAs are regulated after IRI. miRNAs identified as differentially modulated were not derived from either affected by NK cells or lymphocytes infiltrating the renal. Therefore, the results show the miRNA profile identified reflects lymphocyte-independent changes in the renal after IRI. MiR-21 plays a role in several processes that occur as a result of IRI. MiR-21 expression was up-regulated in proliferating tubular epithelial cell (TEC).Citation16
Induction of hypoxia or IR in TEC resulted in up-regulation of miR-21, and an up-regulation of Bcl2. In cultures, knockdown of miR-21 resulted in an increase in apoptosis, whereas over-expression of miR-21 led to decreased apoptosis. The results show that though up-regulation of miR-21 may modulate proliferative responses in TEC by regulating Bcl2 and thereby prevent apoptosis, the level to which miR-21 modulates expression of these gene products, is not sufficient to prevent apoptosis resulting from ischemia. Therefore, miR-21 may play a role in the cellular responses to injury, although it is not sufficient to prevent apoptosis resulting from severe damage.Citation16
TGF-β plays an important role in renal IRI. The studies suggest that miR-21 expression in TEC is induced as a result of TGF-β signaling. Also, in the kidney, TGF-β signaling, as well as hypoxia or ischemia, probably mediates fibrosis through their effect on miR-21 expression. TGF-β affects miR-21 expression by increased processing of pri-miR-21. In TEC, TGF-β stimulation and IR lead to increased processing of pri-miR-21. In renal undergoing IRI, pri-mir-21 was up-regulated at early time-points, although miR-21 was up-regulated at all time points. Thus, the studies suggest a role for miR-21 in renal IRI, up-regulation of miR-21 in vascular smooth muscle cells has been correlated with increases in Bcl2. However, miR-21 expression is inversely associated with Bcl2 expression in breast cancer. These data indicate that the relationship between miRNA expression and function may be cell or tissue type dependent as well as mechanistically distinct.Citation16
Three miRNAs (miR-21, miR-200 and miR-205) are up-regulated following muscle IRI. Ischemia for 1 h is sufficient to induce the expression of miR-21 and miR-200 during the reperfusion phase. Also, 2 h of ischemia are required to induce the expression of miR-205. MiR-200 and miR-205 are regulated after ischemia but their expression pattern changes after reperfusion, indicating their temporal expression during IRI. The expression of miR-21 gradually increases after IRI. These findings imply an important role of miR-21 during IRI. In addition, miR-21 has been reported to have anti-apoptotic properties in cancer cells, and protects against the hydrogen peroxide-induced injury after IR injury.Citation10
MicroRNA-146
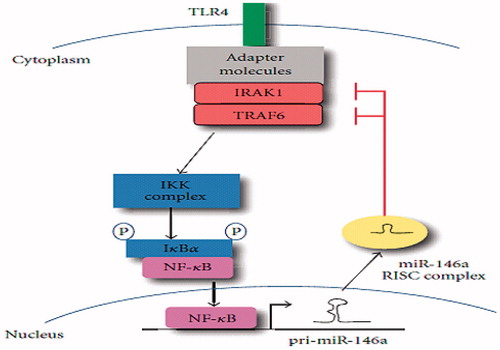
Toll-like receptor 4 (TLR-4) signaling plays an important role in renal IRI and results in NF-κβ activation. MiR-146a expression may lead to down-regulation of TLR signaling which may be required to resolve inflammation and induce repair after IRI.Citation16 Also, the results show that miR-146a/b expression could be induced by stimulation with TNFα and IL-1β, which implies that miR-146 mRNA are expressed in synovial fibroblasts in response to TNFα and IL-1β. Primary-miRNA analysis found elevated miR-146a/b expression in the CKD, and this expression increased with the development of CKD. Thus, miR-146a is a CKD-associated miRNA. Microarray and primary-miRNA expression analyses revealed that miR-146a showed the highest expression level in the kidneys of the aged CKD model. Studies, with real-time PCR analysis, clarified that the expression of miR-146a increased with aging. It is strongly suggested that the main source of the increased miR-146a expression in CKD kidneys is interstitial infiltrating cells, especially those of the monocytic lineage. Therefore, miR-146a is an important regulator of local chronic inflammation.Citation25
miR-146 is a known negative regulator of the type I interferon (IFN) and TLR-4 signaling pathway. Taganov et al.Citation26 reported that miR-146a/b is induced in response to lipopolysaccharide and proinflammatory mediators and miR-146a induction is regulated by NF-κB. They found that miR-146a/b targets were TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1) genes and concluded that miR-146 plays a role in fine-tuning innate immune responses by negative feedback, including down-regulation of TRAF6 and IRAK1 genes ().
Other MiRNAs (miR-29, miR-199a, miR-223, miR-320, and miR-494)
MiRNAs play critical roles in diverse diseases by altering transcription of important genes. The recent studies showed that miR-29 is involved in IR injury; inhibition of miR-29 by anti-sense inhibitors reduced cell death induced by IR injury. Thus, the findings show an anti-apoptotic effect of miR-29 inhibition in IR injury; down-regulation of miR-29 by antagomir limits apoptosis in the risk area in IRI.Citation27
Recent findings suggest regulatory role of miRNAs in divers liver diseases, including hepatitis, hepatic IRI and toxin-induced liver injury. miR-223 has a potential role in liver disease, the expression of this miRNA was observed in liver IR. Ischemic livers were associated with up-regulation of miR-223 expression, also this expression was observed to be positively correlated with the severity of IR injury. Acyl-CoA synthetase long-chain family member 3 (ACSL3) is a member of the enzyme family that catalyzes the synthesis of acyl-CoA using long-chain fatty acids, CoA, and ATP as their substrates. Up-regulation of miR-223 expression in ischemic livers may result in inhibition of ACSL3, thereby reducing synthesis of acyl-CoA. These results provided evidence that miR-223 may be involved in the process of liver IR injury.Citation28
A unique aspect of miRNA function, serving as molecular switches that trigger a rapid change in gene expression in response to a stimulus. For example, miR-199a is sensitive to low oxygen levels in IR and is quickly reduced to undetectable levels, thereby releasing miRNA targets from its inhibitory effect. In addition, after longer periods of ischemia or hypoxia, miR-199a precursor starts to accumulate, suggesting that its transcription and primary transcript processing are unaffected by hypoxia. Recent studies suggest that selective miRNA stability and processing of the stem-loop are subject to modulation in response to external stimuli.Citation29
MiR-320 is an IR-related miRNA. Knockdown of endogenous miR-320 expression reduced apoptosis induced by IR, while overexpression of miR-320 increased sensitivity to IR-triggered cell death. Therefore, at the cellular level, both loss of function and gain of function indicate that miR-320 is a negative regulator of protection against IR injury. HSP20 is one of miR-320 targeted proteins; it is mostly up-regulated in hearts upon ischemic conditions. This suggests that HSP20 may play an important role in cellular stress-resistance and development of tolerance as an adaptive response after exposure to different stimuli. Down-regulation of miR-320 might represent an important adaptive mechanism to up-regulate the expression levels of HSP20 during the IR; previous reports have shown that HSP20 protects against IR, inhibits platelet aggregation and regulates vasorelaxation. Thus, knockdown of miR-320 provides protection against IR-induced apoptosis by targeting HSP20, a well-studied protector.Citation30
The studies demonstrate that increased miR-494 levels protect against IR-triggered injury. miR-494 targeted not only pro-apoptotic proteins, but also anti-apoptotic proteins. MiR-494 may constitute a new therapeutic agent for the treatment of IRI. MiR-494 overexpressing hearts were resistant to IR injury, whereas knockdown of endogenous miR-494 showed the opposite effect. MiR-494 as an anti-apoptotic MiRNA acts via activation of the Akt-mitochondrial signaling pathway. Thus, administration of miR-494 may introduce the newest prospect for the management of ischemic heart disease.Citation31
MiRNAs and nitric oxide synthase
Recent studies propose that whole body heat shock leads to synthesis of several miRNAs, which lead to protection against IR injury. miRNAs induced by IR injury play a major role in protection against IRI; the induced miRNAs cause the up-regulation of endothelial nitric oxide synthase (eNOS), and HSP70 that are implicated in the delayed phase of IRI. These miRNAs can induce HSP70, which may play a role in ischemic tolerance. It is known that miRNAs function as inhibitory mechanisms of gene expression and it is possible that suppression of genes participating in injurious processes during IRI may underlie miRNA-induced protection. The protection observed against IRI suggests that action of several miRNAs (miR-1, miR-21, and miR-24) may have been responsible for the increased expression of HSP70 and eNOS.Citation32 It was reported that ischemic preconditioning up-regulates miR-1, miR-21, and miR-24. Injection of these miRNAs induces eNOS and HSP70. The findings reveal a pivotal role for specific miRNAs in the control IRI-induced cell death. Inducing these miRNAs by pharmacological approaches are safe methods for protection against IR injury.Citation27
Erythropoietin
Erythropoietin (EPO), a glycoprotein, is produced by kidneys. Recombinant human EPO has been used to treat anemia in clinical, and EPO induce cytoprotection via its receptor expressed in tissues against IR injury in different organs including heart, kidney and brain. Endogenous EPO is produced by renal fibroblasts, and EPO receptors are expressed on mesangial, endothelial and TECs. EPO administration before ischemia decreases tubular injury and improves renal function. Also, EPO treatment prior to IR increases Bcl2 protein, reduces tubular epithelial apoptosis and caspase-3 activation, which is associated with HSP70 induction. Caspase-3 plays a major role in inflammation and apoptosis. HSP70, a molecular chaperone, decreases stress-induced aggregation and denaturation of intracellular proteins, which are thought to be the mediators of ischemic tolerance. Also, HSP70 exerts anti-apoptotic effects.Citation33,Citation34
EPO plays a pivotal role in the differentiation, proliferation and maturation of erythroid progenitor cells. Recent researches show that miR-188, miR-210 and miR-362 were highly up-regulated in an EPO-dependent human cell line, UT-7/EPO. In addition, down-regulation of miR-210-induced apoptosis in UT-7/EPO cells. Also, it was recognized that miR-210 was markedly expressed in erythroid cells derived from fetal liver cells. EPO stimulation induced the expression of these miRNAs in UT-7 cells. Thus, these findings showed that the expression of miR-188, miR-210 and miR-362 could be involved in EPO-dependent mechanisms. These miRNAs could be novel members of this erythropoiesis-regulating miRNA family. Also, miR-451 was indicated to accumulate to very high levels in red blood cells and was involved with erythroid maturation. Therefore, the studies on miRNAs in erythroid cells may be useful tools for prognosis and diagnosis in hematological disorders.Citation35
Melatonin
Melatonin (MEL), n-acetyl-5-methoxytryptamine, is secreted by the pineal gland. MEL has been known to act as a synchronizer of the biological clock and has several therapeutic effects, including anti-oxidant, anti-inflammatory effects and immunomodulatory actions. MEL reduces the IR-induced liver, heart, kidney, and brain injury in rats. These effects of MEL are related to scavenging of a variety of toxic oxygen and nitrogen-based reactants and stimulation of anti-oxidative enzymes.Citation36,Citation37 In addition, MEL has an anti-cancer function including suppression of the metabolism of tumor cells and induction of tumor suppressor genes in cancer cell, for example, breast cancer cells. MEL is reported to modify the expression of a number of genes in breast cancer cells, suggesting the pivotal role of MEL in alteration of miRNA expression.
The anti-proliferative effects of MEL have been documented in other tumor types, including ovarian, prostate, and liver cancer. The differential expression of miRNAs is associated with concentration of MEL. Thus, MEL shows differential modulation of miRNA expression according to the concentration. For example, treatment with 1 nM MEL resulted in down-regulation of expression of targeted genes related to cell growth and differentiation and up-regulation of expression of targeted gene related to signal transduction and apoptosis. Therefore, the anti-cancer effects of MEL may involve a change of miRNAs expression.Citation38 Also, miRNA expression can mediate the anti-proliferative action induced by physiological levels of MEL in breast cancer. These results prompt the possible use of expressed miRNAs as new targets for use in research anticancer effects of MEL.Citation38
Conclusion
In kidneys, there are different types of cells which may be affected in diverse renal diseases. However, to understand the pathophysiological role played by one miRNA, it would be necessary to identify the cell type that expresses the miRNA in a disease condition. The model of transgenic animals with conditional overexpression or knockdown of a miRNA might provide the best model to evaluate the function and modulation of miRNA in renal diseases. Identifying the functional significance of a change in miRNA modulation and its target genes under a certain condition can be very difficult because a single protein can be targeted by several miRNAs and a single miRNA can target several proteins. Though we have not yet determined how alterations in the miRNAs observed to be differentially modulated functionally affect the renal as response to injury, we propose that the profile of miRNA expression observed after renal IR injury reflects a survival response. Moreover, although miRNA modulation of renal IR injury is an exciting emerging field of research, it is important to generate a coherent picture of the miRNAs, alterations in renal diseases, their targets, and pathophysiological roles, which provides a comprehensive study of the pathogenesis and leads to the development of new therapeutics. As a note, the majority of miRNAs we identified appear to be differentially expressed, probably to quell the damage response by modulating apoptosis and proliferation and may point us toward new pathways that can be targeted to regulate renal IRI. If the miRNAs we identified as being differentially expressed can be identified to play a role in these processes in vivo, they may represent novel biomarkers of renal IR injury.
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Chatterjee P. Novel pharmacological approaches to the treatment of renal ischemia reperfusion injury: A comprehensive review. Naunyn-Schmiedeberg Arch Pharmacol. 2007;376:1–43
- Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci USA. 2010;107(32):14339–14344
- Wei Q, Bhatt K, He H-Z, Mi Q-S, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia–reperfusion injury. J Am Soc Nephrol. 2010;21(5):756–761
- Zager RA, Johnson AC, Lund S. Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury. Am J Physiol Renal Physiol. 2009;297(4):F961–F970
- Guan Q, Nguan CY, Du C. Expression of transforming growth factor-β1 limits renal ischemia–reperfusion injury. Transplantation. 2010;89(11):1320–1327
- Casey TM, Arthur PG, Bogoyevitch MA. Necrotic death without mitochondrial dysfunction-delayed death of cardiac myocytes following oxidative stress. Biochim Biophys Acta. 2007;1773(3):342–351
- Marambio P, Toro B, Sanhueza C, et al. Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim Biophys Acta. 2010;1802(6):509–518
- Cullingford TE, Wait R, Clerk A, Sugden PH. Effects of oxidative stress on the cardiac myocyte proteome: Modifications to peroxiredoxins and small heat shock proteins. J Mol Cell Cardiol. 2006;40(1):157–172
- Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47(1):5–14
- Hsieh C-H, Jeng JC, Jeng S-F, et al. MicroRNA profiling in ischemic injury of the gracilis muscle in rats. BMC Musculoskelet Disord. 2010;11(1):123
- Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50(3):377–387
- Cheng Y, Tan N, Yang J, et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci. 2010;119:87–95
- Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: Shared themes amid diversity. Nat Rev Genet. 2008;9(11):831842
- Erson A, Petty E. MicroRNAs in development and disease. Clin Genet. 2008;74(4):296–306
- Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009;4(7):1255–1266
- Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci. 2010;107(32):14339–14344
- Yin C, Wang X, Kukreja RC. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia–reperfusion in mice. FEBS Lett. 2008;582(30):4137–4142
- Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: A springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298(5):F1078–F1094
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10(2):126–139
- Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1(3):200–208
- Liang M, Liu Y, Mladinov D, et al. MicroRNA: A new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol. 2009;297(3):F553–F558
- Naesens M, Sarwal MM. Molecular diagnostics in transplantation. Nat Rev Nephrol. 2010;6(10):614–628
- Bhatt K, Mi Q-S, Dong Z. microRNAs in kidneys: Biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol. 2011;300(3):F602–F610
- Cheng Y, Zhu P, Yang J, et al. Ischemic preconditioning-regulated miR-21 protects heart against ischemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. 2010;87(3):431–439
- Ichii O, Otsuka S, Sasaki N, Namiki Y, Hashimoto Y, Kon Y. Altered expression of microRNA miR-146a correlates with the development of chronic renal inflammation. Kidney Int. 2011;81(3):280–292
- Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci. 2006;103(33):12481–12486
- Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-γ agonist protects against myocardial ischemia–reperfusion injury. Cardiovasc Res. 2010;87(3):535–544
- Yu C-H, Xu C-F, Li Y-M. Association of MicroRNA-223 expression with hepatic ischemia/reperfusion injury in mice. Dig Dis Sci. 2009;54(11):2362–2366
- Rane S, He M, Sayed D, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1α and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104(7):879–886
- Ren X-P, Wu J, Wang X, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119(17):2357–2366
- Wang X, Zhang X, Ren X-P, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122(13):1308–1318
- Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104(5):572–575
- Ahmadiasl N, Banaei S, Alihemati A, Baradaran B, Azimian E. Effect of a combined treatment with erythropoietin and melatonin on renal ischemia reperfusion injury in male rats. Clin Exp Nephrol. 2014;18(6):855–864
- Yang B, Hosgood SA, Bagul A, Waller HL, Nicholson ML. Erythropoietin regulates apoptosis, inflammation and tissue remodelling via caspase-3 and IL-1β in isolated hemoperfused kidneys. Eur J Pharmacol. 2011;660(2):420–430
- Kosaka N, Sugiura K, Yamamoto Y, et al. Identification of erythropoietin-induced microRNAs in hematopoietic cells during erythroid differentiation. Br J Hematol. 2008;142(2):293–300
- Ahmadiasl N, Banaei S, Alihemmati A. Combination antioxidant effect of erythropoietin and melatonin on renal ischemia–reperfusion injury in rats. Iran J Basic Med Sci. 2013;16(12):1209
- Ahmadiasl N, Banaei S, Alihemmati A, Baradaran B, Azimian E. The anti-inflammatory effect of erythropoietin and melatonin on renal ischemia reperfusion injury in male rats. Adv Pharm Bull. 2014;4(1):49–54
- Lee SE, Kim SJ, Youn JP, Hwang SY, Park CS, Park YS. MicroRNA and gene expression analysis of melatonin-exposed human breast cancer cell lines indicating involvement of the anticancer effect. J Pineal Res. 2011;51(3):345–352