Abstract
Introduction: The response to hepatitis B vaccine in the dialysis population is reduced compared to the general population. The intradermal (ID) hepatitis B vaccine has been studied as a potential alternative to intramuscular (IM) administration. This alternative route of administration may illicit a response via a distinct immunologic pathway that may help achieve higher seroconversion rates and thus, protection against hepatitis B infection in this vulnerable patient population. Methods: A literature search was performed in January 2015 using Embase, MEDLINE, and the Cochrane Central Register of Controlled Trials with keywords including, hepatitis B vaccines, intradermal, dermal, intracutaneous, dialysis, hemodialysis, continuous ambulatory peritoneal dialysis, CAPD, peritoneal dialysis, renal failure, chronic renal failure, chronic kidney disease, chronic renal insufficiency, End Stage Renal Disease, ESRD, and CKD. Our search strategy was restricted to human studies published in the English language, and additional literature was retrieved by hand-searching bibliographies of relevant articles. Two reviewers (F.Y. and S.G.) independently reviewed abstracts and/or full texts of articles retrieved from the electronic database using the above-mentioned search strategy. Inclusion criteria were as follows: (1) Published, English-language studies performed in the human population, (2) adult patient population (≥18 years of age), (3) randomized trials, (4) patient population must have been unresponsive to a primary IM hepatitis B vaccination protocol, (5) patients must be chronic dialysis patients, either on maintenance hemodialysis or continuous ambulatory peritoneal dialysis (CAPD), (6) studies that compare IM and ID hepatitis B vaccination-associated seroconversion rates, (7) results must be reported as seroconversion rates at 1–3, 6–9, 12, or 20 months post-vaccination, and (8) seroconversion (protective antibody levels) defined as >10 or ≥10 IU/L. Results: Our initial literature review yielded 113 results, of which four were included in our final review. These four prospective trials studied a combined total of 204 dialysis patients. Of these patients, 120 (59%) had received the hepatitis B vaccine intradermally, while 84 (41%) received it intramuscularly. Hepatitis B vaccination type, dose, route, and seroconversion rates were tabulated for each study. Each of the studies used different protocols for patient inclusion, schedule of vaccine administration, and time-points for measuring seroconversion. Seroconversion rates at either 1, 2, 3, 6–9, 12 and/or 20 months were reported. The combined seroconversion rates were 91%, 83%, 86%, 81%, 76%, and 32% at 1, 2, 3, 6–9, 12, and 20 months in the ID group, respectively, and 55%, 72%, 58%, 44%, 24%, and 0% in the IM group, respectively. Chi-square analysis revealed a significantly higher proportion of patients achieving seroconversion in the ID group versus the IM group (p < 0.05). Conclusions: Our review demonstrates that ID hepatitis B vaccination in primary non-responders undergoing dialysis provides an effective alternative to IM vaccination as a means of protection against hepatitis B infection in this highly susceptible population. Additional well-designed, double-blinded, randomized trials are warranted to establish clear guidelines on ID Hepatitis B vaccine dose and duration of vaccination schedule.
Introduction
Hepatitis B infection has been a threat that has largely been removed from the general population due to the implementation of an intramuscular (IM) hepatitis B vaccine schedule in children and adults. However, studies have shown that vaccination with IM injection against hepatitis B has been less effective in the immunocompromised dialysis population.Citation1 Compared with the immunocompetent adult, only 64% of dialysis patients achieve protective antibody levels (defined as ≥10 IU/L) after hepatitis B vaccination.Citation1,Citation2 This is an issue for both patients and healthcare providers given the high-risk environment of the dialysis unit. The risk of acquisition and transmission of Hepatitis B virus among hemodialysis (HD) patients is heightened due to their increased exposure to blood, transfusion requirements and from sharing of dialysis equipment.Citation3,Citation4 Primary hepatitis B infection is often self-limited in the immunocompetent host, but it progresses to chronic infection in two-thirds of immunocompromised patients.Citation5 Chronic disease may later manifest as liver conditions ranging from chronic hepatitis and liver cirrhosis to hepatocellular carcinoma and 15–25% of these patients die prematurely as a result of these conditions.Citation1 A recent retrospective review by Lin et al. also reports non-responsiveness to hepatitis B vaccination as an independent predictor of infection-associated but not all-cause mortality in ESRD dialysis patients, after 5-year follow-up and adjustment for age, diabetes, albumin levels, gender, and dialysis modality.Citation6
Intradermal (ID) hepatitis B vaccination of chronic dialysis patients, as opposed to IM vaccination, has been proposed because of its efficacy, cost-effectiveness, and smaller dose requirements.Citation4,Citation7–11 The efficacy of the ID vaccination may be related to the dense network of immunological dendritic cells located within the dermis of the skin. After administration of the ID vaccination, the antigen is taken up by dendritic cells residing in the dermis, which mature and travel to the regional lymph node where further immunostimulation takes place.Citation12
To date, studies comparing the ID hepatitis B vaccination to the IM injection have proved favorable to the ID route, but no lasting changes have been made to vaccination protocols in light of the new evidence. As the threat of chronic liver disease secondary to hepatitis B infection increases in the high-risk setting of the dialysis unit, clinical interpretation of current studies is needed in order to develop best-practice guidelines.Citation8 The objective of this review is to summarize the available literature on seroconversion rates following ID and IM hepatitis B vaccination in dialysis patients failing to respond to the primary hepatitis B vaccination series.
Methods
Search methods for identification of studies
A literature search was performed in January 2015 using Embase, MEDLINE, and the Cochrane Central Register of Controlled Trials with keywords including, hepatitis B vaccines, intradermal, dermal, intracutaneous, dialysis, hemodialysis, continuous ambulatory peritoneal dialysis, CAPD, peritoneal dialysis, renal failure, chronic renal failure, chronic kidney disease, chronic renal insufficiency, End Stage Renal Disease, ESRD, and CKD. The following search strategy was used: hepatitis B vaccine AND (intradermal OR dermal OR intracutaneous) AND (dialysis OR renal dialysis OR hemodialysis OR continuous ambulatory peritoneal dialysis OR CAPD OR peritoneal dialysis OR renal failure OR chronic renal failure OR chronic kidney disease OR renal insufficiency OR chronic renal insufficiency OR End Stage Renal Disease OR ESRD OR CKD). From the published literature search results, non-English and non-human studies were manually removed. Additional literature, including reviews and textbook chapters, was retrieved by hand-searching bibliographies of relevant articles. Additional information regarding qualitative methods was requested by directly contacting each article’s corresponding author.
Study selection criteria
Two reviewers (F.Y. and S.G.) independently reviewed abstracts and/or full texts of articles retrieved from the electronic database using the above-mentioned search strategy. Inclusion criteria were as follows: (1) Published, English-language studies performed in the human population, (2) adult patient population (≥18 years of age), (3) randomized trials, (4) patient population must have been unresponsive to a primary IM Hepatitis B vaccination protocol, (5) patients must be chronic dialysis patients, either undergoing maintenance HD or peritoneal dialysis, (6) studies that compare IM and ID hepatitis B vaccination-associated seroconversion rates, (7) results must be reported as seroconversion rates at 1–3, 6–9, 12, or 20 months post-vaccination, and (8) seroconversion (protective antibody levels) defined as >10 or ≥10 IU/L.
Assessment of methodological quality
The methodological quality of each included study was evaluated by two authors (F.Y. and S.G.) based on randomization, blinding, allocation concealment, and follow-up completeness, in accordance with Schulz et al.Citation13 These four criteria of methodological assessment were characterized as adequate, unclear, or inadequate, with additional details when available. Any disagreements between the reviewers were resolved by consulting senior author, C.C.
Analysis of seroconversion rates
Patient numbers and their corresponding combined seroconversion rates for each group at each available time-point were calculated.
Results
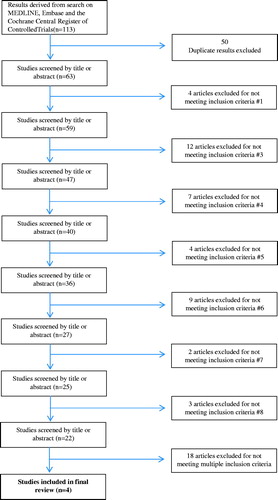
Our initial literature search yielded 113 results, 45 from Embase (1980 to Week 1 2015), 47 from MEDLINE (1946 to Week 3 November 2014), and 21 from the Cochrane Central Register of Controlled Trials (December 2014). Each article abstract was then read to filter for studies that met our inclusion criteria. Fifty studies were excluded from our results as duplicates and 59 were excluded for not meeting one or more of our inclusion criteria. Thus, our analysis of the available literature yielded four articles which met our inclusion criteria and were included in our final review (). All search results and reasons for exclusion are represented fully in Appendix 1. Responses to queries regarding method of randomization and concealment were not received from the corresponding authors of the included articles.
Two of the four trials have described an adequate method of randomization and two trials have an adequate follow-up. The method of randomization, blinding, concealment, and follow-up was unclear in the remaining trials, which create a moderate risk of bias. These elements are important to the integrity of any prospective trial and are summarized in .
Table 1. Methodological quality of included studies.
In these four trials, a combined total of 204 subjects were studied. Of these, 120 (59%) had received the hepatitis B vaccine intradermally, while 84 (41%) received it intramuscularly. The demographics of the patient population included in these trials are described in . There was no significant difference between the number of patients with diabetes in both the IM and ID groups of Barraclough et al. (ID: 10, IM: 7, p = 0.3) and Fabrizi et al. (ID: 8, IM: 5, NS).Citation10,Citation11 The other two included studies did not report diabetes status. Significant differences in age were also not found between groups in Barraclough et al. (ID: 60 ± 15, IM: 54 ± 18, p = 0.2), Micozkadioglu et al. (ID: 60, IM: 55.5, p > 0.05) and Fabrizi et al. (ID: 67.4, IM: 63.1, NS).Citation10,Citation11,Citation14 Dialysis vintage was also not significantly different in 3 of 4 studies, with a mean time on dialysis of 35 months (22–48) in the ID group and 31 months (15–70) in the IM group (p = 0.9) in Barraclough et al., 28 months in the ID group and 29.5 months in the IM group (p = 0.9) in Micozkadioglu et al. and 41 months in the ID group and 19 months in the IM group (p = 0.06) in Fabrizi et al.Citation10,Citation11,Citation13 The fourth study by Radziszewski et al. included patients with a mean time on dialysis of 23 months (1–118). Statistical significance and specific information on dialysis vintage for IM and ID groups were not reported.Citation9
Table 2. Demographics of included studies.
The hepatitis B vaccination type, dose, route, and seroconversion rates were tabulated for each study (). Each of the studies used different protocols for patient inclusion, schedule of vaccine administration and time-points for measuring seroconversion. Seroconversion rates at 1, 2, 3, 6–9, 12 and/or 20 months were reported. The combined seroconversion rates were 91%, 83%, 86%, 81%, 76% and 32% at 1, 2, 3, 6–9, 12, and 20 months in the ID group, respectively, and 55%, 72%, 58%, 44%, 24%, and 0% in the IM group, respectively.
Table 3. Results of included studies.
Radziszewski et al. performed their analysis on 62 patients who were all on maintenance HD for an average of 23 months (range: 1–118).Citation9 Forty-nine patients were included in the ID group and 13 in the IM group. All chosen subjects had been unresponsive to a standard double dose (40 mcg) of Engerix B (SmithKline & Beecham, Brentford, United Kingdom) IM vaccination protocol given at 0, 1, 2 and 6 months. Unresponsive status was determined by anti-HBs levels <9 IU/L in a period of 1–6 months after vaccination. Patients in the ID and IM groups received the same dose of Engerix B vaccine (120 mcg) over a 12-week period. The site of ID and IM injections were not specified. Seroconversion rates at 1, 2, 3, 9, and 12 months post-vaccination for the ID group were 88%, 86%, 88% 84%, and 88%, respectively; and those for the IM group were 85%, 100%, 85% 92%, and 69%, respectively (). Reminder doses were given to 15 patients during the trial, however, specific information regarding the number of doses given and which group these patients belonged to (IM vs. ID) was not given.
Each of the patients considered for inclusion in Fabrizi et al. completed a course of three doses of 40 mcg of recombinant HB vaccine via the IM route, and did not show positive anti-HBs antibody post-vaccination.Citation11 Fifty patients were included in their final analysis, 45 of whom were on chronic maintenance HD and five on continuous ambulatory peritoneal dialysis (CAPD). Twenty-five patients were randomly allocated to the ID group and 25 to the IM group. This is the only article included in our review which included peritoneal dialysis patients in their cohort. Both IM and ID groups were given an equal cumulative dose of 80 mcg of Engerix-B (SmithKline & Beecham, Brentford, United Kingdom). However, the IM schedule ended at 1 month after two doses of 40 mcg, while the ID schedule continued for 16 weeks with 5 mcg weekly. Seroprotective antibody levels were defined as ≥10 IU/L. Seroconversion rates for the ID group were 96% at 1 month, 83% at 3 months, 69% at 6 months, 50% at 12 months, and 30% at 20 months. Seroconversion rates for the IM group at 1, 3, 6, 12, and 20 months were 40%, 44%, 14%, 0%, and 0%, respectively (). No additional doses were given to patients, regardless of antibody level status. Fabrizi et al. was the only study to perform an analysis of costs comparing different vaccination protocols, finding that their IM and ID vaccination protocols saved between $46 and $92 USD, compared to the standard IM re-vaccination protocol.
Barraclough et al. included patients who had anti-HBs antibody titers less than 10 IU/L 1 month after a primary vaccination series of three 40 mcg doses of recombinant hepatitis B vaccine (brand not mentioned) via the IM route at 0, 1, and 6 months.Citation10 Twenty-nine patients in the ID group and 30 patients in the IM group were given the same cumulative dose of 80 mcg. No additional doses of vaccine were administered to members of either group. However, the patients in the ID group received vaccine injections on dialysis days, while the IM group received them on non-dialysis days to “avoid IM injection coinciding with heparin administration”.Citation10 Seroprotective antibody levels were defined as ≥10 IU/L. Seroconversion rates at 2 months post-vaccination were 79% in the ID group and 40% in the IM group ().
Micozkadioglu et al. included patients with anti-HBs antibody titers <10 IU/L after two separate IM vaccination series’ of Genhevac B (Pasteur, Paris, France ) 40 mcg given at 0, 1, 2, and 6 months.Citation14 This is in contrast to the other three studies in this review, which declared non-responder status after one failed IM vaccination series. Of 31 patients included in the final analysis, ID vaccination was administered to 17 patients and IM vaccination to 14 patients. Seroprotective antibody levels were defined as >10 IU/L. Seroconversion rates at 6–9 months post-vaccination were 94% in the ID group and 50% in the IM group (). Additional information on the allocation and demographics of the patient population is represented in and , respectively. Of note, Micozkadioglu et al. were the only study to use Genhevac B (Pasteur, Paris, France) hepatitis B vaccine, which is not a licensed hepatitis B vaccine in the USA. Also, the cumulative doses of the IM group (160 mcg) exceeded the dose of the ID group (80 mcg) by double. An explanation for this difference in dosage was not given.
The presence of side effects after vaccination was noted in two of four studies. Fabrizi et al. reported 5 patients who complained of pain and erythema at the injection site in the first 24 h after vaccination. Of these, two were in the IM group and three in the ID group.Citation11 Barraclough et al. reported that patients in both groups noticed minor symptoms of local pain and/or erythema, but this did not lead to withdrawal or modification of the vaccination schedule in any patients.Citation10 However, the number of patients who experienced side-effects and their group placements were not mentioned.Citation10 Radziszewski et al. report that side effects were not noted in any of their 62 patients who were asked to report the presence of adverse reactions during a 3-day period after each inoculation.Citation9 Micozkadioglu et al. did not mention the side-effect profile after IM or ID vaccination in their patients.Citation14
Discussion
It is clear that ID hepatitis B re-vaccination offers an efficacy advantage over IM vaccination when considering the large percentage of non-responders within the dialysis population. For those in whom IM vaccination has proven successful, no change in vaccine protocol is necessary. However, for non-responders to standard IM injection, alternatives are of paramount importance due to patients’ high risk of acquiring hepatitis B and the clinical significance of progressing disease. Mast et al. show that even among HD patients who respond to vaccine administration, clinically significant hepatitis B infection has been documented in those who did not maintain anti-HBs titers above 10 mIU/mL.Citation15 Our review is the first of its kind to summarize the efficacy of ID hepatitis B vaccination specifically in the non-responsive dialysis population. Moreover, our review demonstrates that ID vaccination is an efficacious and safe alternative to the current protocol of hepatitis B vaccination in non-responders undergoing maintenance dialysis.
Currently, the Centers for Disease Control and Prevention (CDC) recommend hepatitis B vaccination for all susceptible pre-end-stage renal disease and chronic peritoneal and HD patients. Per CDC recommendations, those patients who have anti-HBs levels below10 mIU/mL after primary vaccination series should be re-vaccinated with a second IM vaccination series, consisting of 3–4 doses of hepatitis B vaccine. Patients in whom IM re-vaccination fails to produce protective antibody levels should then be tested for HBsAg. Those patients with positive HBsAg are to undergo appropriate medical management and household, sex, and/or needle-sharing contacts should be identified and vaccinated. HBsAg negative patients should be considered susceptible to hepatitis B infection and counseled about proper precautions to protect against infection.Citation1 No other alternative management is currently in place for patients who are HBsAg negative and do not respond to two IM vaccination series. Thus, ID hepatitis B vaccination provides an attractive alternative for this particular population.
Our analysis demonstrates that the response to ID hepatitis re-vaccination was superior to IM re-vaccination despite differing doses, vaccination schedules, and types of vaccination employed in the four prospective trials. A fully representative comparison of the four studies was not possible due to the heterogeneity of patient population, vaccine type, protocol for both primary vaccination and re-vaccination and time-points of anti-HBs testing after vaccination protocol between studies. However, every effort has been made to analyze the similarities and differences between each of the included studies and represent the conclusions made by each. Though heterogeneous, data from each study were pooled as it was deemed reasonable to combine similar time-points for representation of seroconversion within the IM and ID groups. The components of each study, including vaccine type, protocol, patient demographics, cumulative dosing, and seroconversion rates at different time-points are depicted in and . The evidence obtained from these four studies, although exposed to a moderate risk of bias, shows that ID administration poses a viable and safe alternative to IM administration of hepatitis B vaccination. At 1, 2, 3, 6–9, 12, and 20 months post-vaccination, combined ID seroconversion rates were 91%, 83%, 86%, 81%, and 76%, respectively, and combined IM seroconversion rates were 55%, 58%, 58%, 44%, and 24%, respectively ().
Two studies performed in the non-responsive dialysis population were not included in our review. One study by Chang et al. analyzed the difference between intracutaneous (IC) and IM administration of hepatitis B vaccine in 25 non-responsive HD patients and found a 90% response rate in the IC group versus 57% in the IM group, which support the findings of our review.Citation16 This study was excluded from our analysis because the time-points of reported seroconversion rates were unclear. The other study by Sorkhi et al. compared seroconversion rates after either IM, ID, or subcutaneous (SC) administration of one dose of Engerix B vaccine to a total of 34 HD patients that had antibody levels <100 mIU/mL after three doses of Engerix B vaccine. At 45 days post-vaccination, both the ID and the SC groups had higher rates of seroconversion as compared with the IM group. Six months later, there was a decrease in seroconversion rates at 6 months in the ID and SC groups, which was greater than that in the IM group.Citation17 However, this study described an antibody response of >100 mIU/mL as seroconversion as well as for defining non-responsiveness to primary hepatitis B vaccination series. Therefore, this study was excluded from our review because it did not meet our criterion of seroconversion threshold of ≥10 IU/L.
Practical concerns might be raised regarding the safety and convenience of ID injections revolving around pain caused by hypodermic needles, and inconsistent delivery, even among skilled professionals. Only two of the four studies reviewed reported minimal side effects including mild local pain and/or erythema at the injection site.Citation10,Citation11 While side-effects were not mentioned in Micozkadioglu et al., the remaining study had no reported vaccine side-effects in a total of 62 patients.Citation9 With the development of new ID delivery methods such as the hollow microneedle and needle-free liquid jet injection system, even these concerns are largely eradicated. Kim and Prausnitz even suggest these new ID methods to be so revolutionary as to allow “skin vaccination [to] transition from a topic of immunological interest with limited clinical utility to a viable method of vaccination to increase vaccine immunogenicity and broaden vaccination coverage”.Citation18 A broad review by Bryan et al. of Engerix B, Recombivax HB and the plasma-derived Hepatavax-B vaccinations given via ID injection in reduced doses showed the method to be cost-effective, safe and able to produce impressive percentages of seroconversion; from 49% to 100%.Citation19
Several theories addressing the lack of seroconversion with the IM vaccination in dialysis patients have been proposed, including the possibility that the issue may be inherent to the patient population itself. Whether vaccinated by the ID or IM route, all patients show a decreased rate of seroconversion over time. Radziszewski et al. make the argument for the role of hematological factors contributing to the effectiveness of vaccination, thereby explaining the lack of immune response in the anemic or uremic dialysis patient. No compelling in vivo studies have been published, but an in vitro study by McFaul et al. showed an enhanced secretion of pro-inflammatory cytokines by leukocytes after the addition of and stimulation by purified hemoglobin.Citation20 Factors intrinsic to the pathophysiology of chronic kidney disease may contribute to the poor seroconversion of some patients, and warrant further investigation. Of note, the vaccination of patients against hepatitis B prior to the initiation of renal replacement therapy has been associated with better outcomes, making vaccination an important consideration early in the management of CKD patients.Citation21
A recent article by Eleftheriadis et al. categorizes the factors affecting hepatitis B vaccination in HD patients into patient-associated factors, dialysis-associated factors and vaccine-associated factors.Citation22 Patient-associated factors include acquired immunity disturbances, age, diabetes mellitus, malnutrition, and stage of chronic kidney disease. Three of the four included studies analyzed various demographic characteristics of their patient populations as predictors to seroconversion. All three studies found age, sex, BMI, and duration of dialysis to be insignificant.Citation4,Citation10,Citation14 One study found alcohol abuse, ESRD etiology and diabetes mellitus to be insignificant factors,Citation4 while the other two found dialysis adequacy, laboratory parameters including hemoglobin, albumin and therapy with erythropoietin or erythropoietin stimulating agents to be insignificant factors.Citation10,Citation14 Significant factors included hematocrit, RBC count, and hemoglobin level.Citation9 This finding is in keeping with previous studies showing that anemia may lead to a decrease in immunological function in patients undergoing chronic dialysis.Citation23 Malnutrition and acquired immunity disturbances could not be assessed from these studies. Dialysis-associated factors cited by Eleftheriadis et al. included mode of dialysis (peritoneal dialysis vs. HD), the use of high-flux versus low-flux membranes, treatment with rHuEPO and Vitamin D deficiency. Lastly, vaccine-associated factors included dose and route of hepatitis B vaccination, with dose-doubling and repeated ID vaccination leading to increased seroconversion rates.Citation4,Citation24 In addition, the concomitant administration of immunostimulants such as levamisole, granulocyte macrophage-colony stimulating factor (GM-CSF), thymopentin (TP5), recombinant interferon-alpha-2b (IFN-α2b) is associated with increased response to vaccination. The use of newer hepatitis B vaccinations, including HB-AS02 and HB-AS04, which have been licensed in Europe since 2005 and show promising results, show faster and higher initial response rates, especially with HB-AS04.Citation22 Of note, Eleftheriadis et al. include in their review that “intradermal vaccination could be useful in HD patients [failing] to respond after two series of the recommended IM vaccine schedule”, which supports the findings of this review.Citation22 Also, our included studies did not employ the use of immunostimulants, however, Fabrizi et al. showed via a cost-analysis that the use of low-dose (2.52 × 105 IU) human recombinant interleukin-2 costs $784 USD, recombinant interferon-gamma costs $140 USD for 2 mIU and $73 USD for each dose of thymopentin (TP5). In comparison, a 20 mcg dose of recombinant hepatitis B vaccine administered intradermally costs $23 USD and is a clearly more cost-effective option if it achieves a similar degree and duration of seroconversion.Citation11
Our review adds to a previously published meta-analysis on this topic by Fabrizi et al.Citation25 The current review looked exclusively at prospective trials within the dialysis population, which offers greater insight and clinical strength to the results. Our focus on ESRD patients, rather than the larger CKD population help draw conclusions specific to primary non-responder patients receiving immunization while on maintenance dialysis, whereas previous reviews have included patients of various stages of CKD.Citation25 Publication bias cannot be assessed because fewer than 10 studies have been included in our review. Furthermore, our review focuses on patients who have never had a response to IM vaccination and shows the potency of the ID route in this population. Our results favor an alternative approach to the current protocol of managing those with failed hepatitis B vaccination by clearly showing that dialysis patients unresponsive to IM vaccination have an improved response after ID vaccination. All of the studies in our review demonstrate superior efficacy of the ID hepatitis B re-vaccination versus IM re-vaccination. Both Bommer et al. and Radziszewski et al. agree that at least five doses of vaccine are necessary to obtain a satisfactory level of protection against infection.Citation9,Citation26 Radziszewski et al. conclude that repeated vaccination is required to ensure immunocompromised patients in the dialysis population have consistent protection. In light of this notion, the most cost-effective, safe, and successful approach should be used in the early treatment of all susceptible patients.Citation8 According to our review, ID vaccination is a better option for the management of vaccination against hepatitis B in the non-responder dialysis population; and it is reasonable and appropriate to offer a trial of ID re-vaccination to all dialysis patients who fail to respond to the IM route as standard clinical practice.
Conclusion
Our review suggests that ID vaccination against hepatitis B in non-responders in the dialysis population is an effective alternative to the IM series. The studies analyzed have shown a consistently increased rate of seroconversion and longer maintenance of protective titers after ID vaccination as opposed to IM vaccination. In order to implement change in the current hepatitis B vaccination, recommendations for the non-responding dialysis patient population, additional well-designed, double-blinded, randomized trials are warranted to establish clear guidelines on ID hepatitis vaccine dose and duration of vaccination schedule. Given these results, studies should also be considered to investigate the clinical and cost benefits of ID vaccination as a first-line method for hepatitis B vaccination in the dialysis population.
Declaration of interest
The results presented in this paper have not been published previously in whole or part, except in abstract format. Authors have no conflict of interest.
References
- CDC. Guidelines for Vaccinating Kidney Dialysis Patients and Patients with Chronic Kidney Disease, summarized from Recommendations of the Advisory Committee on Immunization Practices (ACIP). December 2012
- Filippelli M, Lionetti E, Gennaro A, et al. Hepatitis B vaccine by intradermal route in non responder patients: An update. World J Gastroenterol. 2014;20(30):10383–10394
- Snydman DR, Bryan JA, London WT, et al. Transmission of hepatitis B associated with hemodialysis: Role of malfunction (blood leaks) in dialysis machines. J Infect Dis. 1976;134(6):562–570
- Fabrizi F, Dixit V, Messa P, Martin P. Intradermal vs intramuscular vaccine against hepatitis B infection in dialysis patients: A meta-analysis of randomized trials. J Viral Hepat. 2011;18(10):730–737
- Hyams KC. Risks of chronicity following acute hepatitis B virus infection: A review. Clin Infect Dis. 1995;20(4):992–1000. [Review]
- Lin SY, Liu JH, Wang SM, et al. Association of response to hepatitis B vaccination and survival in dialysis patients. BMC Nephrol. 2012;13:97
- Sirsat RA, Shah BV, Nair S, Shetty D, Rodriguest C, Mehta A. Efficacy of low dose intradermal recombinant hepatitis B vaccine with and without immunomodulation in end stage renal disease patients on maintenance hemodialysis. J Assoc Physicians India. 1995;43(3):191–192
- Vlassopoulos D, Arvanitis D, Lilis D, Hatjiyannakos D, Louizou K, Hadjiconstantinou V. Complete success of intradermal vaccination against hepatitis B in advanced chronic renal failure and hemodialysis patients. Ren Fail. 1997;19(3):455–460
- Radziszewski A, Gajda M, Pituch-Noworolska A, et al. The evaluation of the effectiveness of multiple dose intradermal hepatitis B re-vaccination in hemodialyzed patients not responding to standard method of immunization. Przegl Lek. 2007;64(7–8):470–475
- Barraclough KA, Wiggins KJ, Hawley CM, et al. Intradermal versus intramuscular hepatitis B vaccination in hemodialysis patients: A prospective open-label randomized controlled trial in nonresponders to primary vaccination. Am J Kidney Dis. 2009;54(1):95–103. Erratum in: Am J Kidney Dis. 2009;54(2):393. Dosage error in published abstract; MEDLINE/PubMed abstract corrected
- Fabrizi F, Andrulli S, Bacchini G, Corti M, Locatelli F. Intradermal versus intramuscular hepatitis B re-vaccination in non-responsive chronic dialysis patients: A prospective randomized study with cost-effectiveness evaluation. Nephrol Dial Transplant. 1997;12(6):1204–1211
- Sticchi L, Alberti M, Alicino C, Crovari P. The intradermal vaccination: Past experiences and current perspectives. J Prev Med Hyg. 2010;51(1):7–14
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–412
- Micozkadioglu H, Zumrutdal A, Torun D, Sezer S, Ozdemir FN, Haberal M. Low dose intradermal vaccination is superior to high dose intramuscular vaccination for hepatitis B in unresponsive hemodialysis patients. Ren Fail. 2007;29(3):285–288
- Mast EE, Weinbaum CM, Fiore AE, et al., Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of adults. MMWR Recomm Rep. 2006;55(RR-16):1–33; quiz CE1--4. Erratum in: MMWR Morb Mortal Wkly Rep. 2007;56(42):1114
- Chang PC, Schrander-van der Meer AM, van Dorp WT, van Leer E. Intracutaneous versus intramuscular hepatitis B vaccination in primary non-responding hemodialysis patients. Nephrol Dial Transplant. 1996;11(1):191–193
- Sorkhi H, Dooki MR, Ebrahimnejad MS. Low-dose intradermal and subcutaneous versus intramuscular hepatitis B vaccination in primary non-responding hemodialysis patients. J Med Assoc Thai. 2006;89(10):1648–1653
- Kim YC, Prausnitz MR. Enabling skin vaccination using new delivery technologies. Drug Deliv Transl Res. 2011;1(1):7–12
- Bryan JP, Sjogren MH, Perine PL, Legters LJ. Low-dose intradermal and intramuscular vaccination against hepatitis B. Clin Infect Dis. 1992;14(3):697–707
- McFaul SJ, Bowman PD, Villa VM. Hemoglobin stimulates the release of proinflammatory cytokines from leukocytes in whole blood. J Lab Clin Med. 2000;135(3):263–269
- Hashemi B, Mahdavi-Mazdeh M, Abbasi M, et al. Efficacy of HBV vaccination in various stages of chronic kidney disease: Is earlier better? Hepat Mon. 2011;11(10):816–820
- Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I. Factors affecting effectiveness of vaccination against hepatitis B virus in hemodialysis patients. World J Gastroenterol. 2014;20(34):12018–12025
- Yorioka N, Hamaguchi N, Takasugi N, et al. Effect of recombinant human erythropoietin administration on immunological indices in patients undergoing chronic hemodialysis. Nihon Jinzo Gakkai Shi. 1993;35(8):981–988
- Bruguera M, Rodicio JL, Alcazar JM, Oliver A, Del Rio G, Esteban-Mur R. Effects of different dose levels and vaccination schedules on immune response to a recombinant DNA hepatitis B vaccine in hemodialysis patients. Vaccine. 1990;8(Suppl.):S47–S49; discussion S60–S62
- Fabrizi F, Dixit V, Magnini M, Elli A, Martin P. Meta-analysis: Intradermal vs. intramuscular vaccination against hepatitis B virus in patients with chronic kidney disease. Aliment Pharmacol Ther. 2006;24(3):497–506
- Bommer J, Ritz E, Andrassy K, et al. Effect of vaccination schedule and dialysis on hepatitis B vaccination response in uraemic patients. Proc Eur Dial Transplant Assoc. 1983;20:161–168
Appendix 1